While all eyes were turned on carbon dioxide, almost by chance a
few researchers discovered that other gases emitted by human activity
could have serious global impacts. In the 1970s attention centered on damage to the atmosphere's protective ozone layer, and nations combined to diminish the risk. In the mid 1980s scientists realized that other gases, especially methane and nitrates, added together could be as important as carbon dioxide in bringing global warming. The sources and interactions of the gases were multiple and complex, and research brought many surprises; for example, the world's livestock turned out to be a major problem. As methane levels soared ever higher, researchers warned that feedbacks such as increased emissions from warming soils could dangerously accelerate global warming. The warnings had little impact on policy. (This essay is supplementary to the core
essay on The Carbon Dioxide Greenhouse Effect For the most potent
greenhouse gas, water vapor, see the essay on Simple
Models of Climate.)
|
Subsections:
Methane (immediately below), Ozone and CFCs (1970-1980),
Other Gases as a Major Factor (the 1980s), Perils and Policies |
| Methane (1859-1970s) | - LINKS - |
| In 1859 John Tyndall, intrigued by the recently discovered ice ages, took to studying how gases may block heat radiation and thus affect the global climate. Since the work of Joseph Fourier in the 1820s, scientists had understood that the atmosphere might hold in the Earth's heat. The conventional view nevertheless was that gases were entirely transparent. Tyndall tried that out in his laboratory and confirmed it for the main atmospheric gases, oxygen and nitrogen, as well as hydrogen. He was ready to quit when he thought to try another gas that happened to be right at hand in his laboratory: coal-gas. This was a fuel used for lighting (and Bunsen burners), produced industrially by heating coal. It consisted of carbon monoxide (CO) mixed with methane (CH4) and other hydrocarbon gases. Tyndall found that for heat rays, the gas was as opaque as a plank of wood. Thus the industrial revolution, intruding into Tyndall's laboratory in the form of a gas-jet, declared its significance for the planet's heat balance. | Full discussion in |
| Tyndall immediately went on to study other gases, finding that carbon dioxide gas (CO2) and water vapor in particular also block heat radiation. (He could have seen this at the outset if he had noticed a brief publication by an unknown amateur in America, a scientific backwater. Eunice Newton Foote had used a simple device to show that these gases can absorb heat radiation from the Sun.) Tyndall figured that besides water and CO2, "an almost inappreciable mixture of any of the stronger hydrocarbon vapors" would affect the climate.(1*) |
|
| But while CO2 was only a few parts in ten thousand in the Earth's atmosphere, that was still much more than other trace gases. There is so little of Tyndall's "hydrocarbon vapors" in the atmosphere that the most important of them, methane, was not detected there until 1948.(2) Moreover, in most places on a daily basis water vapor outweighs even CO2 for blocking heat radiation. In unraveling the causes of the ice ages or any other climate change, there seemed no reason to look further at methane and the like. For a century nobody paid much attention to anything except water and CO2. | |
| Largely out of simple curiosity about geochemical cycles involving minor carbon and hydrogen compounds, in the 1960s and 1970s scientists cataloged a variety of sources for methane in the atmosphere. It turned out that emissions from biological sources outranked mineral sources. Especially important were bacteria, producing the methane ("swamp gas") that bubbles up in wetlands. That included humanity's countless rice paddies.(3) | |
| These studies, however, gave no reason to think that the gas had any significance for climate change. Thus an authoritative 1971 study of climate almost ignored methane. "To the best of our knowledge," the review concluded, "most atmospheric CH4 is produced [and destroyed] by microbiological activity in soil and swamps." The annual turnover that the experts estimated was so great that any addition from human sources added only a minor fraction. "For this reason, and because CH4 has no direct effects on the climate or the biosphere, it is considered to be of no importance for this report." The authors recommended monitoring the atmospheric levels of the gases SO2, H2S, NH3, and even oxygen, but not methane.(4) There the matter rested through the 1970s. | |
| Ozone and CFCs (1970-1980) TOP OF PAGE | |
| If methane drew little attention, still less went to other trace chemicals in the atmosphere. They were seen as curiosities scarcely worth a scientist's effort. Up to the 1970s, the atmosphere, as one expert later recalled, "was viewed as inert chemically, and for good reason — most of the chemicals known to be present near the surface were essentially inert." The air seemed to be just a simple, robust fluid "that transported pollution away from cities, factories, and fires."(5) A small amount of research did get underway in the 1950s on how various atmospheric chemicals behaved, but only because their interactions were responsible for urban smog. The public had begun to demand action on the smelly and sometimes lethal pollution. Scientists were especially puzzled by the rapidly thickening smog of Los Angeles, so different from familiar coal-smoke hazes. It was a biochemist who finally recognized, by the smog's peculiar odor, what was going on. When the bright Southern California sunshine irradiated automobile exhaust it created a witch's brew of interacting compounds, starting with highly reactive ozone.(6) The scientists who studied ozone chemistry, interested in ground-level pollution, gave no thought to possible connections with global warming. |
|
| The history of climate science is full of unexpected linkages, but perhaps none so odd and tenuous as the events that drew public attention to ozone in the upper atmosphere. It started with concerns over a fleet of supersonic transport airplanes that governments envisioned. Beginning in 1970, a few scientists drew attention to the nitrates (NO, NO2, and in general NOx) that the jet planes would emit in the stratosphere, along with sulfates (SO2) and water vapor. They speculated that the chemical aerosols could stimulate the formation of water droplets, altering cloud cover with unknown effects on climate. Moreover, the chemist Paul Crutzen showed that a single nitrate molecule, reacting again and again in catalytic cycles, could destroy many molecules of ozone.(7) That could be serious, for the wispy layer of stratospheric ozone is all that blocks harmful ultraviolet rays from reaching the Earth's surface. For the first time, a portion of the atmosphere was shown to be chemically fragile, easily changed by a modest addition of industrial emissions. The ozone problem combined with other, weightier arguments to sink the plans for a supersonic transport fleet. |
|
| The new ideas provoked a few scientists to take a look at how the upper atmosphere might be affected by another ambitious project — the hundreds of space shuttle flights that NASA hoped to launch. They found that the chlorine that shuttles would discharge as they shot through the stratosphere might be another menace to the ozone layer. This concern, discussed at a meeting in Kyoto in 1973, helped inspire Mario Molina and Sherwood Rowland look into other chemical emissions from human activities. The result of their calculations seemed fantastic. The minor industrial gases known as CFCs (chlorofluorocarbons) could become a grave threat to the ozone layer. | |
| Experts had thought that the CFCs were environmentally sound. Industry produced the gases in relatively small quantities. And they were very stable, never reacting with animals and plants — which seemed like a point much in their favor. James Lovelock had decided to track these gases in the atmosphere precisely because they were stable markers of industrial activity. His interest arose from meteorologists' concerns about the haze that was marring summers in rural England — was this actually smog produced by industry? Measuring CFCs, which had no source but human industry, seemed a good way to check this. First Lovelock needed to measure the base-level of the gas, far out at sea. Not without difficulty he managed to do this (his proposal for government funds was rejected and he only semi-officially got a spot on a research vessel). As expected, CFCs were everywhere. Not wishing to stir up environmentalists, in 1973 Lovelock remarked that "The presence of these compounds constitutes no conceivable hazard."(8) |
|
| In fact, it was exactly the stability of CFCs that made them a hazard. They would linger in the air for centuries. Eventually some drifted up to a high level where, as Molina and Rowland explained, ultraviolet rays would activate them. They would become catalysts in a process that would destroy ozone, threatening an increase of skin cancer and other dangers. (Back in 1961 veteran meteorologist Harry Wexler had recognized that chlorine atoms could act as catalysts to destroy ozone. A heart attack felled him before he could publish the information, and a decade passed before it was rediscovered. It is a striking demonstration of the meager state of research on atmospheric chemistry — like many other topics related to climate — in the 1960s.)(9*) | |
| When scientists explained the threat to the ozone layer to the public, an agitated controversy broke out over the use of CFCs in aerosol spray cans and the like. The crude but worrisome calculations, and the vehement public response, drove a major expansion of observational and theoretical studies of the stratosphere's chemistry. |
= Milestone |
| If these peculiar gases could do so much to ozone, could they also affect climate? Already in 1973, Lovelock remarked at a scientific conference that CFCs might make a contribution to the greenhouse effect.(10) He followed up by demonstrating that there were unexpectedly high levels of the familiar industrial chemical carbon tetrachloride (CCl4) in the atmosphere, and warned that it was important to unravel the atmospheric chemistry of all chlorine-bearing carbon compounds.(11) | |
| Lovelock's findings, combined with Rowland and Molina's warnings that CFCs would linger in the atmosphere for centuries, provoked a closer look into the question by NASA's Veerabhadran Ramanathan (known to his colleagues as "Ram"). In 1975 he reported that CFCs absorb infrared radiation prodigiously — a single molecule could be 10,000 times as effective as a molecule of CO2. A calculation suggested that CFCs, at the concentrations they would reach by the year 2000 if the current industrial expansion continued, all by themselves might raise global temperature by 1°C (roughly 2°F).(12*) The following year another group published a more elaborate calculation with a simplified model of the atmosphere, admittedly "primitive" but good enough to get a general idea of the main effects. In particular they looked at two chemicals that hardly anybody had thought about, nitrous oxide (N2O) and methane. If the level of both in the atmosphere doubled, they reported, it would raise the temperature another 1°C.(13) Meanwhile Ramanathan's group calculated that ozone too significantly trapped radiation. Keeping its level in the stratosphere high would add to the greenhouse effect.(14) |
|
| All these gases had been overlooked because their quantities were minuscule compared with CO2. For example, an important 1974 report stated categorically that "minor constituents like N2O, CH4, etc. are present in such small concentration that their direct effects are negligible."(14a) Yet there was already so much CO2 in the air that the spectral bands where it absorbed radiation were already quite opaque, so you had to add a lot more of the gas to make a serious difference (for more on this "saturation" see the essay on Basic Radiation Calculations). A few moments' thought would have told any scientist that it was otherwise for trace gases. Each additional wisp of these would help obscure a "window," a region of the spectrum that otherwise would have let radiation through unhindered. But the simple is not always obvious until someone points it out. Understanding took a while to spread. Well into the 1980s, the public, government agencies, and even most scientists thought "global warming" was essentially synonymous with "increasing CO2." Meanwhile, many thousands of tons of other greenhouse gases were pouring into the atmosphere. |
|
| Other Gases as a Major Factor (the 1980s) TOP OF PAGE | |
| In 1980, Ramanathan published a surprising estimate of the contribution to global warming from miscellaneous gases — methane, N2O, and ozone along with CFCs — produced by industry and also by agricultural sources such as fertilizer. He found that these gases might contribute as much as 40% of the total warming caused by gases of human origin (with CO2 responsible for the other 60%). That was highly uncertain, he warned, remarking that his estimate "may become outdated before it appears in print." Scientists were just beginning to work out the complicated chemical interactions among the trace gases and between each gas and sunlight. For example, it had only recently been recognized how much ozone was generated in the air by reactions among other smog chemicals. "The problem," Ramanathan concluded, "because of its potential importance, should be examined in more detail."(15) |
|
| Several years passed without anybody taking up the challenge. It was hard for scientists to conceive that gases whose presence in the atmosphere was barely detectible could have a serious impact on climate. Eventually Ramanathan did the job himself. In 1985 his team published a study of some 30 trace gases that absorbed infrared radiation. These additional "greenhouse gases," they estimated, added together could bring as much global warming as CO2 itself. The announcement shocked the community of climate scientists (for by now the different specialties that dealt with climate followed one another’s work closely enough to form a community).(16) Would the climate changes expected to result from a doubled CO2 level, a level the world might reach a century ahead, in fact come upon them twice as fast — perhaps within their own lifetimes? The next year Robert Dickinson and Ralph Cicerone addressed the question with a calculation based on the new estimate of the effects of all greenhouse gases. They figured that by the year 2050 global temperature could rise several degrees, "and possibly by more than 5°C," if self-reinforcing feedbacks took hold. The 22nd century would be even worse.(17) |
|
| Ramanathan and others argued that the potential for global warming gave reason to restrict production of CFCs. However, most of the scientific and public concern was turning to a more immediate problem, the "ozone hole." This seasonal dearth of protective ozone was discovered over Antarctica in 1985. It seemed likely that CFCs were to blame. Within two years that was demonstrated, when daring flights over Antarctica confirmed new theories of how the chemicals could destroy ozone in very cold air.The threat of increased skin cancer and other direct harm to living creatures now seemed imminent, and gave reason enough to further restrict production of CFCs.(18*) |
<=>Aerosols |
| Appeals from scientists and public activists led to a ground-breaking international agreement, the 1987 Montreal Protocol. It had great success over the following decade in reducing emissions of CFCs at negligible cost. Not until the 21st century, with its far advanced computers and scientific understanding, did people realize the terrible scale of the disaster that had been averted. The ecological damage from the collapse of the ozone layer would have been so enormous that the dying vegetation would have emitted as much CO2 as major nations. That was only a start, for CFCs did have a powerful greenhouse effect all on their own. In 2007 researchers reported that the Montreal Protocol had actually done more to retard global warming than the Kyoto Protocol (the 1997 international agreement on restricting emissions, mainly CO2). If ozone-destroying gases had not been regulated, by the 2080s average global temperature could have climbed something like 4°C, an unthinkable calamity approaching the great extinctions in the geological record. (19) | <=>International |
| For other emissions such as sulfates and nitrates, scientific and public attention again focused on short-term local harms, the foul smog and acid rain. Some researchers pointed out, however, that these chemicals could affect climate indirectly by forming aerosols that would alter cloud cover. The pollution studies were rapidly building a stock of scientific information about the complex chemistry of the atmosphere, and it seemed increasingly relevant to climate researchers. So did the unsettling news that a gas like ozone, which significantly influenced the planet's radiation balance, could go through large swings. The groups who were constructing complex computer models of climate began worrying how to incorporate atmospheric chemistry as yet another factor in their systems. |
|
| After Ramanathan identified methane as a significant greenhouse gas, studies of its role in global carbon cycles accelerated. During the 1980s, scientists came to see that although the methane in the air comes largely from plants and animals, that did not mean human effects were negligible. For humanity was transforming the entire global biosphere. Specialists in obscure fields of research turned up a variety of biological methane sources that were rapidly increasing. The gas was abundantly emitted by bacteria found in the mud of rice paddies and burped up from the guts of cud-chewing cows, among other places. Especially intriguing was methane from the guts of termites: an early experiment on one species of termite suggested they might be extremely important. (Later work with other species lowered their significance; it turned out that wetlands were the largest natural source of methane, with termites a distant second producing only one-fifth to one-tenth as much.)(19a) And what about accelerated emissions from the soil bacteria as well as termites that proliferated following deforestation and the advance of agriculture? Moreover, natural biological activity could be altered by the rise of CO2 levels and by global warming itself, making for complicated and enigmatic feedbacks. |
|
| The importance of all this was driven home by a tentative 1981 report that methane in the atmosphere was increasing at an astounding rate, perhaps 2% a year. The following year, a study of air bubbles trapped in ice drilled from the Greenland icecap confirmed that methane was climbing. The climb, radically different from any change that could be detected in past millennia, had started in the 16th century and accelerated wildly in recent decades.(20) By 1988, painstaking collection of air samples at many remote locations gave an accurate measure of the recent rise. The actual rate of increase was about 1% a year, bringing a shocking 11% increase of methane in the past decade alone. Since there was still not much methane in the atmosphere, each additional molecule of methane would have a greenhouse effect many times that of a molecule of CO2. In addition, some of the methane was converted into CO2 and water vapor in the stratosphere, where they would exert their own greenhouse effects. (Taking these and other atmospheric interactions into account, it was later calculated that over the span of a century, additional methane would be tens of times more effective per molecule in producing global warming than additional CO2. See discussion below.) It seemed likely that the rising methane level was already having a measurable impact.(21*) | This raised alarming new possibilities for potentially catastrophic feedbacks. Particularly ominous were the enormous quantities of carbon atoms locked in the strange "clathrates" (methane hydrates) found in the muck of seabeds around the world. Clathrates are ice-like substances with methane imprisoned within their structure, kept solid only by the pressure and cold of the overlying water. A lump of the stuff brought to the surface will fizz and disintegrate, and meanwhile a match can set it aflame. When it became apparent how widespread the clathrates are, they attracted close study as a potentially lucrative source of energy. In the early 1980s, a few scientists had pointed out that if a slight warming penetrated the sediments, clathrates might melt and release colossal bursts of methane and CO2 into the atmosphere. That would bring still more warming.(22*) |
|
| The importance of methane became clearer as more cores were drilled from the ice of Greenland and Antarctica, revealing changes in the levels of gases in the atmosphere back through previous glacial periods. Measurements published in 1988 showed that over hundreds of thousands of years, methane had risen and fallen in step with temperature. The level had been a factor of two higher in warm periods than in glacial periods. Perhaps this was due to variations in how much gas was generated by bacteria in wetlands? Or by abrupt releases from undersea clathrates? For whatever reason, there was evidently some kind of feedback between temperature and the level of methane in the atmosphere, a feedback that might gravely accelerate any global warming.(23) |
|
| In 1988 Ramanathan remarked dryly, "the greenhouse theory of climate change has reached the crucial stage of verification." If the predictions were valid, he said, the rise in trace gases together with CO2 would bring a warming unprecedented in human history. He expected it would become apparent before the year 2010.(24) | |
| Perils and Policies TOP OF PAGE | |
| Attention to gases other than CO2 continued to grow. Ozone holes in the stratosphere over the poles each winter drove home the idea that even small concentrations of some industrial emissions could have powerful effects. On the other hand, the public easily misunderstood the issues (an environmental activist recalled, "I once heard the head of the Environmental Protection Agency totally confuse the climate issue and the ozone depletion issue.") Out of public view, experts delved into the chemical interactions among ozone, nitrates, water vapor, and so forth in every level of the atmosphere from the ground up. Ingenious and difficult computer modeling showed that the concentration of one type of chemical altered the concentration of others, so that the indirect action of a gas could be even greater than its direct greenhouse effect. For example, carbon monoxide (CO) does not intercept much heat radiation by itself, but the massive amounts of the gas that humanity was emitting did alter the levels of methane and ozone. The community of climate scientists could reach no consensus on how serious these complex indirect effects were, and from this point on, the question drove extensive research.(25) | |
| Methane got special attention, for it offered some of the most peculiar and unsettling possibilities, such as increased emission from wetlands as the climate warmed. An especially huge reservoir of carbon is locked up in organic compounds in the permanently frozen peat (permafrost), often many meters deep, that underlies Arctic tundras. Around 1990, scientists began to wonder what would happen if a warming climate turned more of the upper layers to marsh. Would biological activity explode in the endless expanses of sodden tundra, with microbes emitting enough methane to accelerate global warming? One of the scientists, Richard Harriss, argued that monitoring methane emissions from tundra could give an early warning of enormous changes. "The danger of a thermal runaway caused by CH4 release from permafrost is minor," another expert remarked, "but real."(26) |
|
| Measurements were scanty. But the number of publications on permafrost emissions rose exponentially, from almost none in 1990 to more than 60 a year around 2010. For example, in one especially well-studied Swedish bog, researchers reported an increase in methane emissions from 1970 to 2000 of at least 20 percent, and perhaps much more. By 2006 the thawing of large areas of permafrost was visibly underway in many Arctic regions, presumably emitting ever more methane (and equally significant amounts of carbon dioxide). There was good reason to expect that much more would thaw by the end of the century. In 2010 two scientists surveyed their colleagues who published on permafrost, getting 41 responses — there was a substantial community now. "Our collective estimate," they reported, "is that carbon will be released more quickly than models suggest, and at levels that are cause for serious concern." A 2015 review confirmed that permafrost would make a substantial contribution to global warming, although not enough to overshadow fossil fuel emissions. |
|
| Methane, an expert lamented, "has so many kinds of sources and sinks... You have to look at it like you are a detective trying to solve a criminal mystery." For example, initially everyone assumed that methane emissions from wetlands like tundra were annually recycled, the classic textbook paradigm of a "carbon cycle" with no net change in the atmosphere. The belief reflected traditional confidence in a stable “balance of nature.” But in the late 1990s, radiocarbon measurements of methane bubbling up in Siberian lakes found much of it was ancient — as the mud got warmer, it was emitting carbon laid down tens of thousands of years ago. Another source: in 2017, improved methods for measuring emissions from trees pointed to tropical forests as an increasing source of methane, perhaps comparable to tundra. Another: analysis of carbon isotopes in the 2010s indicated that the rise at that time was largely of recent biological origin, most likely exhaled by microbes in the wetlands that cover large regions in the tropics. And back to tundra: an analysis of thousands of measurements found a distinct increase in Arctic wetlands methane emissions in the first two decades of the century.(27) |
|
| Worse, a 2005 study of the complex chemical interactions within the atmosphere itself calculated that adding methane was even more powerful in bringing greenhouse warming than previous studies had estimated. It also seemed increasingly likely that clathrates in the warming seabed would release massive amounts of the gas, although (good news for once) that would probably take thousands of years. Any of these processes might eventually leave the planet stuck more or less permanently with a climate unlike any that had been seen for many millions of years.(27a) |
|
| Back in 1986, Dickinson and Cicerone had carefully separated the temperature changes that gases might ultimately cause from their immediate and direct physical influence on radiation. They called these direct influences "thermal trappings" — what later came to be called "radiative forcings."(28) Unlike the ultimate global temperature with its complex feedbacks, the physical forcings could be calculated in a straightforward and reliable way. That made it easier to compare the consequences of changes in the different agents — not only different gases but also aerosols, cloud cover, changes in land vegetation, the Sun's radiation itself, and so on. This subtle but important shift in approach increasingly took hold over the following decade. |
|
| For the 1990 report of the Intergovernmental Panel on Climate Change (IPCC), scientists had aboriously worked out a way to explain forcings that would be more useful for policy decisions: the "Global Warming Potential (GWP)" of a gas or other influence. This number included not only the direct effects of a gas on radiation, but also how long the gas would linger in the atmosphere. Calculating that was fiendishly difficult, because the gases affect one another through chemical interactions. The levels of methane and ozone, for example, are tightly linked. When a pulse of methane is introduced into the atmosphere, oxidation that produces ozone and other reactions destroy half of the methane in seven years, whereas CO2 lingers for centuries. Over a 20-year time frame a methane molecule has some 80 times the GWP of a CO2 molecule, but over a hundred-year span the ratio is only about 30 (These numbers are methane’s “Carbon Dioxide Equivalent” in another newly minted terminology. The figures given here are from calculations published in 2021; earlier figures were lower.) The GWP values would eventually become important for major policy decisions, such as whether to replace coal in power plants with "natural gas" (that is, fossil gas, mainly methane). Discussions could become confused and even acrimonious when people failed to specify their time scale. | <=>International |
| That pushed into the very center of policy-making the fact that additions of some long-lingering trace gases had a potential for warming, molecule for molecule, hundreds or thousands of times stronger than additional CO2.(29) In particular, although the current greenhouse effect from N2O was not very large, studies found that the gas would remain in the atmosphere for a century or more, with some 300 times the Global Warming Potential per molecule compared with CO2. And the level was soaring, thanks to emissions from fertilizers and cow manure. Climate scientists had never given this gas as much attention as they gave to methane, with its fascinating biological feedbacks. But by the ea rly 21st century, N2O had become become another significant greenhouse gas. | |
| Many scientists now believed that the effects of nitrates had been
seriously underestimated. Indeed, replacing fossil fuels with "biofuel"
manufactured from corn might increase global warming, thanks
to the emissions from soil bacteria stimulated by the fertilizers
used to grow the corn. The more scientists studied the emissions of N2O and other nitrogen compounds, the more confused
they got. Not only was it hard to measure how much was emitted, but
the compounds reacted in complicated ways with smog chemicals, ozone,
methane and CO2. Meanwhile nitrogen compounds
fertilized plants and ocean plankton, helping them to take up carbon — although the plankton also emitted significant N2O). Some of the interactions would result
in more greenhouse gas emissions, while others removed greenhouse gases and would have a net cooling effect. Overall, however, the record in ice cores indicated that N2O, like methane, amplified climate change, perhaps because a warming pulse brought additional emission from microbes.(30) |
|
| Additional problems kept cropping up, even with the simplest of gases. Around 2003 atmospheric chemists turned their attention to proposals that were circulating to convert the world to a "hydrogen economy," where hydrogen would replace fossil fuels for many purposes. At first the scientists' concern focused on how hydrogen-fueled cars would emit less nitrates from their tailpipes. Through a sequence of chemical interactions, that would increase the lifetime of methane in the atmosphere. This seemed a minor matter, but later studies found complexities. Hydrogen in the atmosphere could feed interactions that would increase not only methane but the greenhouse gases ozone and water vapor. According to one estimate, hydrogen had about twelve times the "global warming potential" of CO2 over a 100-year period. That was several times better than methane, but could hydrogen be distributed in the same pipes as the fossil "natural" gas? Massive use of hydrogen might not retard global warming at all, unless leakage of the gas into the atmosphere was rigorously controlled | |
| In analyzing the complicated chemical interactions among all the various trace gases and between the gases and aerosols, computer models offered only limited help. A 2006 survey found many differences in how models handled the problem, with some models afflicted by elementary errors of chemistry or computer coding. The best that could be said was that about half the models agreed reasonably well with observations and with one another, so that "some confidence can be placed in their predictions." The uncertainties made it hard to come up with defensible policies.(30a) |
=>Aerosols |
| Experts now agreed that sound policy should take into account all the potential causes of warming. To take one surprising example, leaks of methane from gas pipelines turned out to add significantly to global warming. However, the headlong rise of methane in the atmosphere seen in the 1970s and 1980s slowed in the 1990s to a more sedate pace. The reasons were unclear (the collapse of the Soviet Union's economy? efficiencies in production and distribution of the gas? the draining of wetlands? droughts?). After 2000 the methane level did not rise at all. Since the CO2 level continued to rise, and the Montreal agreement had brought the production of CFCs to a halt, in the first decade of the new century CO2 was responsible for some three-quarters of the ongoing warming (up from roughly half in the 1980s). Other gases (and aerosols) were often overlooked in public debates, and even in much of the expert policy discussion. As one policy expert sighed, in negotiations "CO2 sucks all the oxygen out of the room."(31) | |
| Around 2007, however, methane emissions resumed their ominous climb. By 2015 it was clear that the rise was not only steep but accelerating, threatening to speed up global heating. By the early 2020s the methane level was rising at double the rate of the previous decade. Scientists advanced a variety of possible explanations for the resurgence of the gas. For example, was the rapid growth of natural gas production by hydraulic fracturing ("fracking") leaking a sizable amount of methane into the atmosphere? Other likely sources were the proliferation of livestock to meet a surging global demand for meat and newly opened seams in the expanding Chinese coal mines (to the surprise of experts, coal mines turned out to be a major source of methane). Some speculated that changes in the chemistry of the atmosphere itself were inhibiting the normal gradual destruction of CH4 molecules there. | |
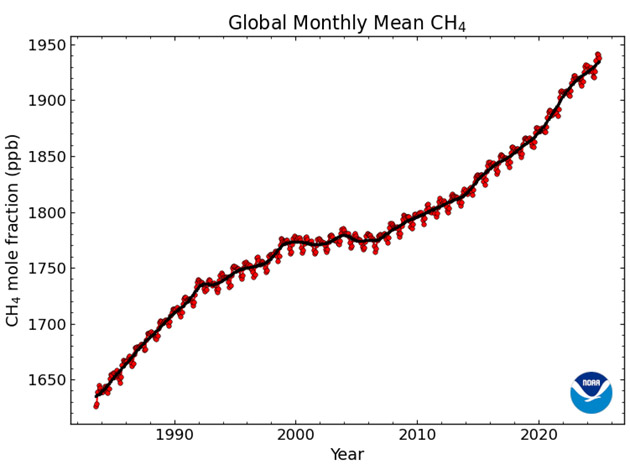 |
Rise of methane gas in the atmosphere |
| Still more ominously, the warming climate could be stimulating increased emissions from microbes in wetlands from the Arctic to the tropics — a feedback cycle with no clear termination. Measurements of carbon isotopes continued to say that most of the new rise was of biological origin, presumably microbes. It was an open question how much of this came from human activities; it was possible, a research team warned, that "a large-scale reorganization of the natural climate and biosphere is under way." As one science journalist put it, the proposed explanations for the methane rise "range from the troubling to the truly hair-raising." Even more bluntly, a scientific reviewer admitted that "catastrophic emissions cannot be ruled out." | <=>Rapid change |
| Monitoring emissions became an important activity; satellite overviews were checked against field data from teams looking into various types of farm and wilderness, not to mention airplanes spying on oilfields. Aggressive steps to cut back inefficient releases of the gases might be the most cost-effective of all ways to reduce the risk of harm from global warming. Regulation of methane leakage from fossil fuel wells and pipelines — which measurements showed was much worse than the industry claimed — became a contentious political issue.(32) | |
| Even more difficult, indeed too difficult for politicians to touch, were the rapidly rising nitrate emissions. Nitrous oxide was the third most abundant greenhouse gas in the atmosphere after CO2 and methane (leaving aside water vapor). The main source of this, and other nitrogen compounds, was fossil fuels, whether burned or converted to fertilizers. As usual, there were complications. Some nitrates promoted aerosol haze that reflected sunlight. Nitrogen compounds could also engage in atmospheric chemistry that reduced methane, along with other complex effects. The net short-term effect of the emissions on global temperature was probably not heating but cooling. Over the long run, however, the steady buildup of atmospheric nitrous oxide, emitted by the fertilizers that were indispensable for global agriculture, would certainly add significant heating. And climate change itself, as it warmed soils and promoted wildfires, could increase the nitrate emissions.(33) |
|
| One area where progress seemed possible was domesticated livestock. When researchers added up the methane burped from the stomachs of ruminants. plus the emissions from their manure and from fertilizing the crops that fed them, they found a surprisingly large factor in the greenhouse equation. It was another way humanity had become a geological force — the mass of our livestock was now more than half of the total mass of all the mammals on the planet (humans and their pets make up most of the rest, with the wild mammals from mice to whales less than 5%). Persuading people that it was healthy to eat less meat would be a significant step toward slowing climate change. | |
| Another and surprising issue was the gases known as HFCs (hydrofluorocarbons), developed to replace the banned ozone-destroying CFCs. Production of the gases for air conditioning and refrigeration was soaring, accelerated by global warming itself —and it turned out that HFCs, while harmless to ozone, were powerful greenhouse gases. Governments hesitated to impose restrictions on the industries producing the new gases, but the need was inescapable. Diplomats struggled to extend the Montreal Protocol, now understood as not only a treaty that protected ozone but a treaty that protected the climate. In 2016 they succeeded in phasing out HFCs (the "Kigali amendment" to the Montreal Protocol). |
<=>Government |
| To make a really big difference in restraining future warming, the fastest way would be to restrain the rise of methane — which by one calculation was responsible for nearly a third of the total rise in global temperature since the industrial revolution. The gas was finally getting serious attention, driven less by new scientific discoveries than by rapid advance in technologies for detecting methane emissions. Major emitters could increasingly be identified and publicly shamed. The IPCC, in its 2021 recommendations to policymakers, gave nearly as much attention to methane as to CO2 itself. Serious discussion of restrictions got underway, with various groups of nations or corporations pledging to restrict their emissions. The political and diplomatic problems would always be as complicated and frustrating as the chemistry of the gases themselves.(34*) |
|
RELATED:
Home
The Carbon Dioxide Greenhouse Effect
Biosphere: How Life Alters Climate.)
| NOTES |
1. The first component of the coal-gas that Tyndall measured in pure form was what he called "olefiant gas," now called ethylene or ethene (C2H4). He subsequently measured "carbonic acid," then a common term for what is now called carbon dioxide, and went on to other hydrocarbons including perfumes such as pachouli and lavendar — but not methane. Today "carbonic acid" means H2CO3. At the end of the century Arrhenius still wrote "carbonic acid" for CO2. On Foote see note in the essay on "The Carbon Dioxide Greenhouse Effect." Tyndall (1863b); Tyndall (1861); Tyndall (1873a), quote p. 40. BACK
3. A pioneer especially for rice paddies was Koyama (1963); wetlands: Ehhalt (1974). BACK
4. Wilson and Matthews (1971), p. 242. BACK
5. Cicerone (1999), p. 19, see also H. Schiff's comments, p. 115. BACK
7. Crutzen (1970) calculated that even small amounts of nitrates could be important as catalysts; this was independently and explicitly linked to supersonic transports and ozone damage by Johnston (1971). BACK
8. Lovelock et al. (1973); wryly quoted by Lovelock himself, Lovelock (1974), p. 293; on motives and funding Lovelock (2000), ch. 8. BACK
9. At this point the compounds were called, more precisely, chlorofluoromethanes. Molina and Rowland (1974) (submitted in June); that "the oxides of chlorine... may constitute an important sink for stratospheric ozone" was independently worked out in Stolarski and Cicerone (1974) (submitted in January) but the consequences were not grasped — the first journal to which the paper was submitted rejected it when a reviewer declared the idea was "of no conceivable geophysical consequence"; Cicerone (2003); see also Cicerone et al. (1974) (submitted in September); for discussion, Gribbin (1988). Wexler: Fleming (2010), pp. 219-21. On the discovery see Crutzen (1995), who remarked: "if the chemical industry had developed organobromine compounds instead of the CFCs... then without any preparedness, we would have been faced with a catastrophic ozone hole everywhere and at all seasons during the 1970s, probably before the atmospheric chemists had developed the necessary knowledge to identify the problem... mankind has been extremely lucky." BACK
12. Ice-albedo feedback, he added, could give considerably greater warming in arctic regions. Ramanathan (1975). BACK
13. Their best guess was 0.7°C for N2O, 0.3°C for methane, and 0.1°C for ammonia. Wang et al. (1976). BACK
14. Ramanathan et al. (1976). BACK
14a..World Meteorological Organization (1975a) BACK
15. Ramanathan (1980), quote p. 269. BACK
16. Ramanathan et al. (1985); for a comment see Bolin (2007), p. 37. BACK
17. Dickinson and Cicerone (1986), quote p. 109. BACK
18. Farman et al. (1985); Susan Solomon and, independently, Michael McElroy and Steven Wofsky explained that the unexpected factor destroying ozone was catalysis on the surface of ice crystals in high clouds. For history and scientific references, see Roan (1989); Christie (2000), and reporting by Richard Kerr in Science magazine from 1987. Roan (1989), see pp. 92, 195. BACK
19.Could have climbed: Velders et al. (2007); Young et al. (2021), Garcia et al. (2012). BACK
19a. Fraser et al. (1986). BACK
20. Rasmussen and Khalil (1981a); see Rasmussen and Khalil (1981b); Craig and Chou (1982). BACK
21. Blake and Rowland (1988). Since methane drops out of the atmosphere faster than CO2, timescale matters in comparing the two. Over a 20-year time frame, the IPCC's 2013 report figured a methane molecule has some 80 times the "global warming potential" of a CO2 molecule. Over a hundred-year span the ratio is roughly 30. BACK
22. To be precise, the sediments would release methane, some of which would convert to CO2. "A potential does exist for significant positive feedback" from Arctic Ocean clathrates, warned Bell (1982), who was stimulated by a 1980 paper presented by Gordon J. MacDonald, see MacDonald (1980). BACK
23. For the last glacial period see Stauffer et al. (1988); Raynaud et al. (1988); for a 160,000 year record Chappellaz et al. (1990); Nisbet (1990a). BACK
24. Ramanathan (1988), quote p. 293. BACK
25.Totally confuse: J.G. (Gus) Speth, 2008 preface to Woodwell et al. (1979), pdf online here, p. 3. Isaksen and Hov (1987); the greenhouse effect of carbon monoxide was therefore perhaps greater than that of N2O. Derwent (1990) is cited as a pioneer by Le Treut et al. (2007), see pp. 108-9. For a summary, see IPCC (2001a), p. 256 and passim. BACK
26. Kvenvolden (1988); Harriss et al. (1992); Harriss (1993). Minor but real: Nisbet (1989). BACK
27. Publication statistics: Kuhry et al. (2010).Swedish bog: Christensen et al. (2004). Rößger et al. (2022) found a long-term~2% increase /year at a locality in Siberia. See also Walter et al. (2006). Survey: Schuur and Abbott (2011). 2015 review: Schuur et al. (2015). "Detective:" Edward Dlugokencky, quoted in Leslie Hook and Chris Campbell, "Methane Hunters: What Explains the Surge in the Potent Greenhouse Gas?," Financial Times, Aug. 23, 2022, online here. Siberian lakes: Zimov et al. (1997). Trees: Pangala et al. (2017); biological origin: Feng et al. (2022), Zhang et al. (2023). Boreal-arctic: Yuan et al. (2024). BACK
27a. Methane: Shindell et al. (2005), Keppler et al. (2006). Clathrates: Archer and Buffet (2005). BACK
28. Dickinson and Cicerone (1986). BACK
29. For GWP see IPCC (1990a), section 2.2.7, IPCC (2021a), table 7.15. BACK
30. Rodhe (1990) calculated that the contribution of an N2O molecule to global warming is 300 times that of a CO2 molecule. Underestimate, biofuels: Crutzen et al. (2008).Ice cores: Flückiger et al. (1999). Models survey: Eyring et al. (2006), see also Doherty (2009). .BACK
30a. Hydrogen: Prather (2003), Tromp et al. (2003), Schultz et al. (2003), Derwent et al. (2006) . "The impact of a H2 economy on the global CH4 budget is likely to be small, except for the feedback between reduced oxidizing capacity (via NOx reduction) and CH4 lifetime," according to IPCC (2007b), p. 547, but 15 years later, "the net climate benefit of a future hydrogen economy is unknown over the near to medium term," Ocko and Hamburg (2022), see also Bertagni et al. (2022). Warming potential: Warwick et al. (2023),q.v. for an optimistic view on hydrogen, Sand et al. (2023). Models survey: Eyring et al. (2006), see also Doherty (2009). .BACK
31.Bousquet (2006); Dlugokencky et al. (2009); Bloom et al. (2010); Simpson et al. (2012); Worden et al. (2017). "Sucks:" David Doniger, quoted by Andrew C. Revkin, "Ozone Solution Poses a Growing Climate Threat," June 22, 2009, online here. BACK
32. Nisbet et al. (2019); Saunois et al. (2016), Saunois et al. (2020); tropical wetlands: Feng et al. (2022), Zhang et al. (2023), Michel et al. (2024). "Reorganization:" Nisbet et al. (2023). "Hair-raising:" "The Methane Mystery," The Economist 427, no. 9089 (April 28, 2018), pp. 71-72, see Tollefson (2022); "catastrophic:" O'Connor et al. (2010). Leakage greater: Alvarez et al. (2018); US emissions were found to be some three times the government's estimate, according to Sherwin et al. (2024).For recent methane trends see the NOAA Global Monitoring Laboratory. BACK
33.Gong et al. (2024). Increased emissions: Wang et al. (2023). BACK
34. Wild mammal biomass (roughly half in the oceans): Bar-On et al. (2018). HFCs: Velders et al. (2009); IPCC (2021b), paragraph D.1; see Andersen et al. (2022) for a list of important papers. Nearly a third of the rise: IEA (International Energy Agency), "Global Methane Tracker 2022," online here; for detection see, e.g., Lauvaux et al. (2022)..BACK
copyright © 2003-2025 Spencer Weart & American Institute of Physics