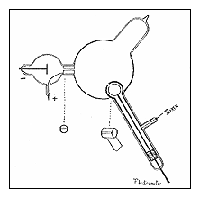
Apparatus used in
Thomson's first
experiment. |
 o atoms have parts? J.J. Thomson suggested that they do. He advanced the idea that cathode rays are really streams of very small pieces of atoms. Three experiments led him to this.: o atoms have parts? J.J. Thomson suggested that they do. He advanced the idea that cathode rays are really streams of very small pieces of atoms. Three experiments led him to this.:
 irst, in a variation of an 1895 experiment by Jean Perrin, Thomson built a cathode ray tube ending in a pair of metal cylinders with a slit in them. These cylinders were in turn
connected to an electrometer, a device for catching and measuring electrical charge. Perrin had found that cathode rays deposited an electric charge. Thomson wanted to see if, by bending the rays with a magnet, he could separate the charge from the rays. He found that when the rays entered the slit in the cylinders, the electrometer measured a large amount of negative charge. The electrometer did not register much electric charge if the rays were bent so they would not enter the slit. As Thomson saw it, the negative charge and the cathode rays must somehow be stuck together: you cannot separate the charge from the rays. irst, in a variation of an 1895 experiment by Jean Perrin, Thomson built a cathode ray tube ending in a pair of metal cylinders with a slit in them. These cylinders were in turn
connected to an electrometer, a device for catching and measuring electrical charge. Perrin had found that cathode rays deposited an electric charge. Thomson wanted to see if, by bending the rays with a magnet, he could separate the charge from the rays. He found that when the rays entered the slit in the cylinders, the electrometer measured a large amount of negative charge. The electrometer did not register much electric charge if the rays were bent so they would not enter the slit. As Thomson saw it, the negative charge and the cathode rays must somehow be stuck together: you cannot separate the charge from the rays.
|
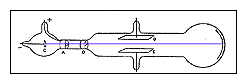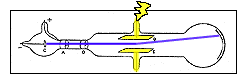
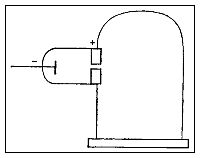
Thomson's apparatus
in the second
experiment. |
 ll attempts had failed when physicists tried to bend cathode rays with an electric field. Now Thomson thought of a new approach. A charged particle will normally curve as it moves through an electric field, but not if it is surrounded by a conductor (a sheath of copper, for example). Thomson suspected that the traces of gas remaining in the tube were being turned into an electrical conductor by the cathode rays themselves. To test this idea, he took great pains to extract nearly all of the gas from a tube, and found that now the cathode rays did bend in an electric field after all. ll attempts had failed when physicists tried to bend cathode rays with an electric field. Now Thomson thought of a new approach. A charged particle will normally curve as it moves through an electric field, but not if it is surrounded by a conductor (a sheath of copper, for example). Thomson suspected that the traces of gas remaining in the tube were being turned into an electrical conductor by the cathode rays themselves. To test this idea, he took great pains to extract nearly all of the gas from a tube, and found that now the cathode rays did bend in an electric field after all.
|
"What are these particles?
Are they atoms, or
molecules, or matter in a still
finer state of subdivision?" |
|
 homson concluded from these two experiments, "I can see no escape from the conclusion that [cathode rays] are charges of negative electricity carried by particles of matter." But, he continued, "What are these particles? are they atoms, or molecules, or matter in a still finer state of subdivision?" homson concluded from these two experiments, "I can see no escape from the conclusion that [cathode rays] are charges of negative electricity carried by particles of matter." But, he continued, "What are these particles? are they atoms, or molecules, or matter in a still finer state of subdivision?"
|
One of the tubes used
in Thomson's third
experiment. |
 homson's third experiment sought to determine the basic properties of the particles. Although he couldn't measure directly the mass or the electric charge of such a particle, he
could measure how much the rays were bent by a magnetic field, and how much energy they carried. From this data he could calculate the ratio of the mass of a particle to its electric charge (m/e). He collected data using a variety of tubes and using different gases. homson's third experiment sought to determine the basic properties of the particles. Although he couldn't measure directly the mass or the electric charge of such a particle, he
could measure how much the rays were bent by a magnetic field, and how much energy they carried. From this data he could calculate the ratio of the mass of a particle to its electric charge (m/e). He collected data using a variety of tubes and using different gases.
|

J.J. Thomson
experimenting, in the
Cavendish Lab. |
 he
results were astounding. Just as Emil Wiechert had reported
earlier that year, the mass-to-charge ratio for cathode rays turned out
to be far smaller than that of a charged hydrogen atom--more than one
thousand times smaller. Either the cathode rays carried an enormous charge
(as compared with a charged atom), or else they were amazingly light relative
to their charge. he
results were astounding. Just as Emil Wiechert had reported
earlier that year, the mass-to-charge ratio for cathode rays turned out
to be far smaller than that of a charged hydrogen atom--more than one
thousand times smaller. Either the cathode rays carried an enormous charge
(as compared with a charged atom), or else they were amazingly light relative
to their charge.
 he
choice between these possibilities was settled by Philipp
Lenard. Experimenting on how cathode rays penetrate gases, he showed
that if cathode rays were particles they had to have a mass very much
smaller than the mass of any atom. The proof was far from conclusive.
But experiments by others in the next two years yielded an independent
measurement of the value of the charge (e) and confirmed this
remarkable conclusion. he
choice between these possibilities was settled by Philipp
Lenard. Experimenting on how cathode rays penetrate gases, he showed
that if cathode rays were particles they had to have a mass very much
smaller than the mass of any atom. The proof was far from conclusive.
But experiments by others in the next two years yielded an independent
measurement of the value of the charge (e) and confirmed this
remarkable conclusion.
|
| "We have in the cathode rays matter in a new state." |
|
 homson boldly announced the hypothesis that "we have in the cathode rays matter in a new state, a state in which the subdivision of matter is carried very much further than in the ordinary gaseous state: a state in which all matter... is of one and the same kind; this matter being the substance from which all the chemical elements are built up." homson boldly announced the hypothesis that "we have in the cathode rays matter in a new state, a state in which the subdivision of matter is carried very much further than in the ordinary gaseous state: a state in which all matter... is of one and the same kind; this matter being the substance from which all the chemical elements are built up."
|
|
|
Table of Contents: Exhibit Home
J.J. Thomson
Mysterious Rays
1897 Experiments 
Corpuscles to Electrons
Legacy for Today
More Info |
 Corpuscles to Electrons Corpuscles to Electrons |






 Corpuscles to Electrons
Corpuscles to Electrons