Alpha Particles and the Atom
Rutherford at Manchester, 1907–1919
Ernest Rutherford discovered the nucleus of the atom in 1911. We read this in textbooks and in popular writings. But what does that statement mean? Geographical discovery usually means that one sees a place for the first time. But can discovery be the same for a realm hidden from sight? One cannot see an atom in that sense. So this hints that perhaps the story of the discovery of the nucleus was more complicated. The story as it unfolded in Rutherford's lab at the University in Manchester revolved around real people. It involved frustrations and triumphs. It involved hard work and perplexity and inspiration.
 When Rutherford became professor at Manchester in 1907, he found modern labs for both teaching and research. At the urging of his predecessor, Arthur Schuster, over £40,000 was raised to endow the physics program. For comparison, Rutherford's generous salary was £1,600/year. Credit: From the book: The physical laboratories of the University of Manchester: a record of 25 years' work by the University of Manchester, Manchester: At the University Press, 1906. AIP Emilio Segrè Visual Archives, Brittle Books Collection.
When Rutherford became professor at Manchester in 1907, he found modern labs for both teaching and research. At the urging of his predecessor, Arthur Schuster, over £40,000 was raised to endow the physics program. For comparison, Rutherford's generous salary was £1,600/year. Credit: From the book: The physical laboratories of the University of Manchester: a record of 25 years' work by the University of Manchester, Manchester: At the University Press, 1906. AIP Emilio Segrè Visual Archives, Brittle Books Collection.
Rutherford arrived in Manchester in the summer of 1907, months before the university's term began. He had been named Langworthy Professor of Physics, successor to Arthur Schuster (1851–1934), who retired at age 56 to recruit Rutherford. Schuster had built a modern physics building, hired Hans Geiger, Ph.D. (1882–1945) because of his experimental skill, and endowed a new position in mathematical physics to round out a full physics program. Rutherford entered the center of the physics world. Researchers came to him by the dozen.
Rutherford arrived with many research questions in mind. He was not done with the puzzles of the decay families of thorium, radium, etc., but he was passing much of this work to Boltwood, Hahn, and Soddy. Boltwood and Hahn both worked with Rutherford in Manchester, Boltwood in 1909–1910 and Hahn in 1907–1908. Rutherford was gradually turning his attention much more to the α (alpha), β (beta), and γ (gamma) rays themselves and to what they might reveal about the atom. That is, he was leaving radio-chemistry to others and turning to physics.
 Rutherford always gathered a group of bright young researchers around him. In this group photo of 1910 are Ernest Marsden and Hans Geiger. Front and center are Professors Schuster and Rutherford, and centered in the rear is William Kay, the talented and helpful laboratory steward. Credit: J.B. Birks, ed., Rutherford at Manchester (London: Heywood & Co., 1962), opposite p. 38.
Rutherford always gathered a group of bright young researchers around him. In this group photo of 1910 are Ernest Marsden and Hans Geiger. Front and center are Professors Schuster and Rutherford, and centered in the rear is William Kay, the talented and helpful laboratory steward. Credit: J.B. Birks, ed., Rutherford at Manchester (London: Heywood & Co., 1962), opposite p. 38.
Rutherford's early team at Manchester included Geiger and William Kay (1879–1961), junior laboratory assistant since 1894. Rutherford promoted Kay to laboratory steward in 1908, to manage lab equipment and to aid him in his research. In 1957, Kay thought back to his youth with Rutherford in an interview. The language is quaint, but the description is as close to Rutherford's approach as we get. The questioner was Samuel Devons (1914–2006), who was one of Rutherford's last students in the 1930s.
[Devons] “When you were here [in Manchester], during this period... did Rutherford actually make any apparatus himself?”
[Kay] “No, no, no, no. We used to, I used to set up nearly all his apparatus. You know, when he did his work, you know, oftener than not, he used to tell me and we did a rough experiment, re...”
[D.] “Did he sketch out what he wanted?”
[K.] “Well, he'd tell you what he wanted, roughly, you see, but he'd let you make what you wanted, you see, he'd tell you what he was going to do, which was very good, you see. It gives you......... it learnt you a lot and you knew what to do and what not to do. And then we would do a rough experiment, and get one or two curves you see, and then straight away button it on to somebody else to do the real work, and that's how he did his........ attacked these little things, you see.”
[D.] “He tried them out himself first?”
[K.] “He'd try a rough experiment himself on the little things, d'you see, and then he'd turn it over on to somebody...” (Quoted in Hughes, p. 104)
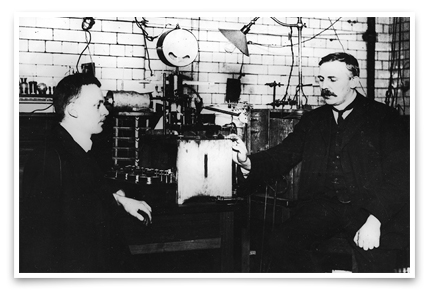 Hans Geiger was Rutherford’s main partner in alpha-ray research from 1907 to 1913. Together they developed several ways to detect alpha rays. They proved alpha rays are doubly-ionized Helium nuclei. Ca. 1908. Credit: AIP Emilio Segre Visual Archives, Physics Today Collection.
Hans Geiger was Rutherford’s main partner in alpha-ray research from 1907 to 1913. Together they developed several ways to detect alpha rays. They proved alpha rays are doubly-ionized Helium nuclei. Ca. 1908. Credit: AIP Emilio Segre Visual Archives, Physics Today Collection.
Rutherford and Hans Geiger worked closely in 1907 and 1908 on the detection and measurement of α particles. If they were to use α particles to probe the atom, they had first to know more about these particles and their behavior. Rutherford had tried and failed back at McGill to count α particles.
A year later in Manchester, he and Geiger succeeded with two methods of observing α particles. The first method involved scintillations excited by α particles on a thin layer of zinc sulfide. They observed these through a microscope and counted the scintillations at different angles of dispersion. They also developed an "electrometer" that could demonstrate the passage of an individual α particle to a large audience. The instrument, which evolved into the "Geiger counter," had a partially evacuated metal cylinder with a wire down its center. They applied a voltage between the cylinder and the wire high enough almost to spark. They admitted α particles through a thin mica window, where these particles collided with gasses, producing gas ions. These then collided with other molecules and produced more ions, and so on. Each α particle produced a cascade of ions, which partially discharged the cylinder and indicated the passage of an α particle. Geiger and Rutherford published several articles in 1908 and 1909 on these methods and their use.
Rutherford wrote to Henry Bumstead (1870–1920), an American physicist, on 11 July 1908:
Geiger is a good man and worked like a slave. I could never have found time for the drudgery before we got things going in good style. Finally all went well, but the scattering is the devil. Our tube worked like a charm and we could easily get a throw of 50 mm. for each particle. ... Geiger is a demon at the work of counting scintillations and could count at intervals for a whole night without disturbing his equanimity. I damned vigorously and retired after two minutes. (Quoted in Eve, p. 180.)
Although Rutherford suspected as early as 1906 that α particles were helium atoms stripped of their electrons, he demanded a high standard of proof. One kind of experiment was not enough. One kind of detector was not enough. He wanted more proof. For this, Rutherford desired "big voltages" and big electromagnets to divert α particles, but this method was not yet ripe. Lab steward William Kay recalled in the cited oral history interview that Rutherford in 1908 insisted that strong electric and magnetic fields were needed to measure more directly the charge and mass of the α and β particles:
And that's what he was after all the time. That's what he got at Cambridge [after 1919], which we never got here, you see, because we'd got no money. (Hughes, “William Kay,” 2008, pp. 109–110.)
Kay said Rutherford wanted a big, water-cooled magnet, but that he “dropped it like a hot cake” when he learned its cost. So he needed a new line of attack. The new line was very simple, a chemical procedure mixed with physics. For this work Rutherford recruited Thomas Royds (1884–1955), who had earned his Physics Honours degree in 1906. They collected α particles in a sealed glass tube, compressed them, and passed an electric spark through. They studied the emitted light in a spectroscope and found it to be identical to the spectrum of helium. Within a few months, Rutherford was awarded the Nobel Prize for Chemistry, "for his investigations into the disintegration of the elements, and the chemistry of radioactive substances." (Nobel citation) Rutherford and Royds had established the identity and primary properties of α particles. Rutherford next turned his attention to using them to probe the atom.
The autumn of 1908 began an important series of researches. Geiger had been passing beams of α particles through gold and other metallic foils, using the new detection techniques to measure how much these beams were dispersed by the atoms in the foils. Geiger thought Ernest Marsden (1889–1970), a 19-year-old student in Honours Physics, was ready to help on these experiments and suggested it to Rutherford. Since Rutherford often pushed third-year students into research, saying this was the best way to learn about physics, he readily agreed.
 This sketch, from Geiger and Marsden's 1909 article, shows a conical glass tube full of "radium emanation" (radon), closed off at B with a thin pane of mica. This was their source of alpha (α) particles. S was a zinc-sulfide screen, which scintillated when struck by an α particle. P was a lead screen, which blocked any α particles from travelling directly to the zinc-sulfide screen. RR was a foil (or foils) of different metals (including gold) which "diffusely reflected" incident α particles. Geiger and Marsden observed the resulting scintillations through an observing microscope, M. Credit: H. Geiger and E. Marsden, "On a Diffuse Reflection of the α-Particles," Proceedings of the Royal Society,1909, 82:495–500.
This sketch, from Geiger and Marsden's 1909 article, shows a conical glass tube full of "radium emanation" (radon), closed off at B with a thin pane of mica. This was their source of alpha (α) particles. S was a zinc-sulfide screen, which scintillated when struck by an α particle. P was a lead screen, which blocked any α particles from travelling directly to the zinc-sulfide screen. RR was a foil (or foils) of different metals (including gold) which "diffusely reflected" incident α particles. Geiger and Marsden observed the resulting scintillations through an observing microscope, M. Credit: H. Geiger and E. Marsden, "On a Diffuse Reflection of the α-Particles," Proceedings of the Royal Society,1909, 82:495–500.
Geiger and Marsden began with small-angle dispersion and tried various thicknesses of foils, seeking mathematical relationships between dispersion and thickness of foil or number of atoms traversed. Marsden later recalled that Rutherford said to him amidst these experiments: "See if you can get some effect of alpha-particles directly reflected from a metal surface." (Reported by Marsden in Birks, 1962, p. 8). Marsden doubted that Rutherford expected back scatter of α particles, but as Marsden wrote
...it was one of those 'hunches' that perhaps some effect might be observed, and that in any case that neighbouring territory of this Tom Tiddler's ground might be explored by reconnaissance. Rutherford was ever ready to meet the unexpected and exploit it, where favourable, but he also knew when to stop on such excursions. (Birks, 1962, p. 8)
This was Rutherford's playful approach in action. His students and others tried out his ideas, many of which were dead-ends. This idea to look for backscattering of α particles, however, paid off. Rutherford wrote:
Experiment, directed by the disciplined imagination either of an individual or, still better, of a group of individuals of varied mental outlook, is able to achieve results which far transcend the imagination alone of the greatest philosopher. (Quoted in Eve, 1939, Frontmatter)
Sometime later in 1908 or 1909, Marsden said, he reported his results to Rutherford. Rutherford recalled this a little differently:
I remember ...later Geiger coming to me in great excitement and saying, 'We have been able to get some of the α-particles coming backwards...' It was quite the most incredible event that has ever happened to me in my life. It was almost incredible as if you fired a 15-inch shell at a piece of tissue paper and it came back and hit you. (Rutherford, 1938, p. 68)
Human memory is fallible. Whether Marsden or Geiger told Rutherford, the effect was the same. Rutherford said they should prepare a publication from this research, which they submitted in May 1909. Moreover, this started Rutherford thinking toward what ultimately, almost two years later, he published as a theory of the atom.
What was Rutherford doing for the rest of 1909 and all of 1910? For one thing, his close friend Boltwood was in Manchester for the academic year working with Rutherford on radioactive decay products of radium. He was also reviewing and speaking on earlier ideas about atomic structure. Most importantly, he was taking the phenomenon of the scattering of α particles apart systematically and testing each piece. Rutherford did not have his bold idea — the nuclear atom — instantly, but he came to it gradually by considering the problem from many sides.
In the autumn of 1910 he brought Marsden back to Manchester to complete rigorous experimental testing of his ideas with Geiger. They re-established rates of emission and the ranges of α particles by radioactive sources and they re-examined their statistical analyses. Rutherford tried to reconcile scattering results with different atomic models, especially that of J.J. Thomson, in which the positive electricity was considered as dispersed evenly throughout the whole sphere of the atom.
 A page of Rutherford's early, undated (1910 or 1911), rough notes. The first few lines read: "Theory of structure of atom. Suppose atom consists of + charge ne at centre & of – charge as electron distributed throughout sphere of radius r." He then roughed out ideas about the calculation of the force of deflection on a charged particle passing close to this charged center. Credit: J.B. Birks, ed., Rutherford at Manchester (London: Heywood & Co., 1962), p. 70.
A page of Rutherford's early, undated (1910 or 1911), rough notes. The first few lines read: "Theory of structure of atom. Suppose atom consists of + charge ne at centre & of – charge as electron distributed throughout sphere of radius r." He then roughed out ideas about the calculation of the force of deflection on a charged particle passing close to this charged center. Credit: J.B. Birks, ed., Rutherford at Manchester (London: Heywood & Co., 1962), p. 70.
At some point in the winter of 1910–1911, Rutherford worked out the basic idea of an atom with a "charged center." As Geiger and Marsden pointed out in their 1909 article:
If the high velocity and mass of the α-particle be taken into account, it seems surprising that some of the α-particles, as the experiment shows, can be turned within a layer of 6 x 10-5 cm. of gold through an angle of 90°, and even more. To produce a similar effect by a magnetic field, the enormous field of 109 absolute units would be required. (Birks, p. 179)
Rutherford concluded in his May 1911 paper that such a remarkable deviation in the path of a massive charged particle could only be achieved if most of the mass of, say, an atom of gold and most of its charge were concentrated in a very small central body. Note: at this point in 1911, Rutherford did not call this a "nucleus."
You need Flash Player installed to listen to this audio clip.
The first public announcement of the nuclear theory by Rutherford was made at a meeting of the Manchester Literary and Philosophical Society, and he invited us young boys to go to the meeting. He said he’d got some interesting things to say and he thought we’d like to hear them. We didn’t know what it was about at that time. The older people in the laboratory did, of course Geiger and Marsden knew because they were already doing the experiments. In fact, unless they had done some which were sufficient to be decisive, Rutherford never mentioned it publicly. And, of course, Darwin knew about it much earlier. But that must have been early in 1911, and we went to the meeting and he told us. And he mentioned then that there was some experimental evidence which had been obtained by Geiger and Marsden. He did not, as far as I remember, say more about the results than that they were quite decisive. And, as I said before, he would never have made a public announcement of that kind if he hadn’t had good evidence. And that is one of the characteristics that runs through all Rutherford’s work, particularly all his work up to the end of the Manchester period. If you look at some of his papers in the early days — I call McGill the early days — he was quite convinced that the alpha particles were atoms of helium, but he never said that in those words. He always said they were either atoms of helium or molecules of hydrogen or perhaps he may have said something else of that weight. It was quite characteristic of him that he would never say a thing was so unless he had experimental evidence for it that really satisfied him.
In fact, Rutherford was exceedingly cautious in drawing conclusions about this central charge: “A simple calculation shows that the atom must be a seat of an intense electric field in order to produce such a large deflexion at a single encounter.” (Birks, p. 183). He worked out quickly and roughly that several quantitative relationships should be true if this basic theory were correct. First, the number of α particles scattered through a given angle should be proportional to the thickness of the foil. Second, that number should be proportional to the square of the nuclear charge. Lastly, it should be inversely proportional to the fourth power of the velocity of the α particle. These three ideas laid out the experimental program of Geiger and Marsden for the next year.
You need Flash Player installed to listen to this audio clip.
Rutherford’s interest was then almost entirely in the research. He had done very little teaching in McGill. He was research professor. I suppose he gave some lectures but it would have been very few. And his interest was quite naturally on the research side. He did give some lectures, but elementary lectures, the kind of thing you would expect a man to know before he came to the University. They were the lectures to the engineers. They were a rowdy lot and Rutherford could keep them under control. There was perhaps only one other man in the department who could have done it, and he (Rutherford?) enjoyed them because he was able to show them the very interesting experiments one can perform in elementary courses.
It's often been said to me that Rutherford was a bad lecturer. I never heard such nonsense. It is quite true that on occasion he would be a bit dull, a bit mixed up, but that was only on very rare occasions. There were other occasions when he was really most stimulating. There was a tremendous enthusiasm about him.
Rutherford entertained the possibility that the charged center is negative. That sounds odd today, so what made it reasonable? First, it wasn't very different from Thomson's model. Second, since Rutherford knew that α particles carry a double + charge, he thought this might act the same way the Sun does on a comet sweeping near it. It would slingshot the α particle around and back towards its source. He also considered a nearly forgotten model suggested by Japanese physicist Hantaro Nagaoka (1865–1950) — the Saturnian model. Nagaoka and Rutherford were in contact in 1910 and 1911 and Rutherford mentioned Nagaoka's model of "a central attracting mass surround by rings of rotating electrons" (Birks, p. 203). The end result in this critical Rutherford paper, however, was Rutherford's announcement that whether the atom were a disk or a sphere, and indeed whether the central charge were positive or negative, would not affect the calculations. Rutherford was always careful not to claim more than his results could support.
 In Rutherford’s now-famous paper of May 1911 on the scattering of alpha particles by gold foil, he included this sketch of the hyperbolic path of a particle. Credit: E. Rutherford, "The Scattering of α and β Particles by Matter and the Structure of Matter," Philosophical Magazine, 1911, 21:669–688.
In Rutherford’s now-famous paper of May 1911 on the scattering of alpha particles by gold foil, he included this sketch of the hyperbolic path of a particle. Credit: E. Rutherford, "The Scattering of α and β Particles by Matter and the Structure of Matter," Philosophical Magazine, 1911, 21:669–688.
Rutherford did see possible tests of the nature of the central charge. The absorption of β particles, he said, should be different with a negative center versus a positive one. A positive center would explain the great velocity that α particles achieve during emission from radioactive elements. But these were only hints.
 This schematic represents the refined experiments of 1912–13 undertaken by Geiger and Marsden. R was the source of alpha particles, E was the gold foil, and M was the microscope rotatable around a vertical axis centered on the gold foil. Alpha particles from the source passed through the diaphragm D, were scattered by the foil, and were observed as scintillations on the screen S. Geiger and Marsden observed the angles of scattered particles by rotating the microscope-screen assembly. Credit: H. Geiger and E. Marsden, “The Laws of Deflexion of α Particles through Large Angles,” Philosophical Magazine, 1913, 25:604–623.
This schematic represents the refined experiments of 1912–13 undertaken by Geiger and Marsden. R was the source of alpha particles, E was the gold foil, and M was the microscope rotatable around a vertical axis centered on the gold foil. Alpha particles from the source passed through the diaphragm D, were scattered by the foil, and were observed as scintillations on the screen S. Geiger and Marsden observed the angles of scattered particles by rotating the microscope-screen assembly. Credit: H. Geiger and E. Marsden, “The Laws of Deflexion of α Particles through Large Angles,” Philosophical Magazine, 1913, 25:604–623.
Geiger and Marsden did indeed work systematically through the testable implications of Rutherford's central charge hypothesis. The first major publication of their results was in German in the Proceedings of the Vienna Academy of Sciences (Sitzungberichte der Wiener Akademie der Wissenschaften) in 1912. This 30-page version was followed by one in English in 1913 in the Philosophical Magazine: "The Laws of Deflexion of α Particles through Large Angles" The English version is the better known. Slight differences between the two led one historian to suggest that Rutherford decided in favor of a positively charged center by August 1912 (Trenn, 1974). Rutherford's other team members, especially Charles Galton Darwin (1887–1962), H.G.J. Moseley (1887–1915), and Niels Bohr (1885–1962) figured prominently in the ultimate establishment of Rutherford's nuclear atom.
 The young Henry G.J. Moseley, in the Balliol-Trinity Laboratory, Oxford, ca. 1910. Later that year, Moseley began research in Rutherford's Manchester lab. His brilliant career was cut short in combat in World War I. Credit: University of Oxford, Museum of the History of Science, courtesy AIP Emilio Segrè Visual Archives, Physics Today Collection.
The young Henry G.J. Moseley, in the Balliol-Trinity Laboratory, Oxford, ca. 1910. Later that year, Moseley began research in Rutherford's Manchester lab. His brilliant career was cut short in combat in World War I. Credit: University of Oxford, Museum of the History of Science, courtesy AIP Emilio Segrè Visual Archives, Physics Today Collection.
The ‘Great War’ totally disrupted work in Rutherford's Manchester department. Bohr returned to Denmark. Marsden accepted a professorship in New Zealand. Moseley died in the Battle of Gallipoli. James Chadwick (1891–1974), who was working with Geiger at the Technical University of Berlin when war broke out, spent several years interned in the Ruhleben camp for prisoners of war. Other students went off to war, too, and Rutherford devoted considerable energy to mobilizing science for the war effort and specifically to anti-submarine techniques.
 Niels Bohr first worked with Rutherford at
Niels Bohr first worked with Rutherford at Manchester in 1912. This photo shows the young Niels and Margrethe Bohr, ca. 1914, when Bohr succeeded Charles Galton Darwin as the Schuster Reader in Mathematical Physics at Manchester. Credit: AIP Emilio Segre Visual Archives, Margrethe Bohr Collection.
Against this distracted background, Rutherford and his lab steward, William Kay, began in 1917 to explore the passage of α particles through hydrogen, nitrogen, and other gases. When the Great War ended, Ernest Marsden briefly helped with the tedious scintillation observations that provided clues to the nature of the nucleus. Rutherford reported the tentative results of these extensive experiments in 1919. Rutherford placed a source of radium C (bismuth-214) in a sealable brass container, fitted so that the position of the source could be changed and so that different gases could be introduced or a vacuum produced, as desired. The α particles traversed the interior of the container and passed through a slit, covered by a silver plate or other material, and hit a zinc sulfide screen, where a scintillation was observed in a darkened room. When hydrogen gas was introduced into the container and care was taken to absorb the α particles before they hit the screen, scintillations were still observed. Rutherford posited that as the α particles traversed the hydrogen gas, they occasionally collided with hydrogen nuclei. As Rutherford wrote, this produced “swift hydrogen atoms” which were mostly projected forward in the direction of the α particles’ original motion.
Rutherford had several subtle questions in mind during these experiments, mostly concerned with the nature of the nucleus. He asked his colleague Darwin to analyze these collisions based on a ‘simple theory’ of elastic collisions between point nuclei repelled according to an inverse square law, the α particles carrying a charge of 2 times that of an electron (and of opposite sign) and the hydrogen nuclei 1 times. Darwin found that all α particles approaching within 2.4x10-13 cm would produce a ‘swift hydrogen atom.’ This simple theory, however, predicted far fewer accelerated hydrogen atoms than were observed in the experiments.
Rutherford rejected explanations of this variance based on different charges on the particles or other laws than inverse square laws. Rather, he concluded that for distances on the order of the diameter of the electron, ‘the structure of the helium nucleus can no longer be regarded as a point…’. He posited that the helium nucleus (α particle) has a complex structure of four hydrogen nuclei plus two negatively charged electrons. (We would say it is composed of two protons.) Rutherford concluded that deformation of complex nuclei during collisions was a more likely explanation, the variation of the forces between the nuclei varying in a complex way on close approach.
Taking into account the intense forces brought into play in such collisions, it would not be surprising if the helium nucleus were to break up. No evidence of such a disintegration…has been observed, indicating that the helium nucleus must be a very stable structure.
We must remember that Rutherford could not directly observe the structure of the nucleus, so his conclusions were tentative. Nevertheless, he was openly considering the possibilities of a complex nucleus, capable of deformation and even of possible disintegration. These thoughts shaped this intense period of experimental researches.