|
 X-rays
and Uranium Rays
X-rays
and Uranium Rays
 ARIE
CURIE'S CHOICE of a thesis topic was influenced by two recent
discoveries by other scientists. In December 1895, about six months
after the Curies married, German physicist Wilhelm Roentgen discovered
a kind of ray that could travel through solid wood or flesh and
yield photographs of living people's bones. Roentgen dubbed these
mysterious rays X-rays, with X standing for unknown. In recognition
of his discovery, Roentgen in 1901 became the first Nobel laureate
in physics. ARIE
CURIE'S CHOICE of a thesis topic was influenced by two recent
discoveries by other scientists. In December 1895, about six months
after the Curies married, German physicist Wilhelm Roentgen discovered
a kind of ray that could travel through solid wood or flesh and
yield photographs of living people's bones. Roentgen dubbed these
mysterious rays X-rays, with X standing for unknown. In recognition
of his discovery, Roentgen in 1901 became the first Nobel laureate
in physics.
|
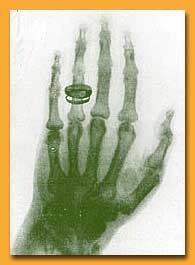 |
| One of Roentgen's first X-ray
photographs -- a colleague's hand (note the wedding ring). The
revelation of X-rays fascinated the public and deeply puzzled
scientists |
|
 |
| Henri Becquerel, discoverer of uranium radiation.
Although he tried to help the Curies solve their financial problems
and advance their careers, the relationship eventually soured--as
sometimes happens with scientists touchy about sharing credit
for discoveries. |
|
In early 1896, only
a few of months after Roentgen's discovery, French physicist Henri
Becquerel reported to the French Academy of Sciences that uranium
compounds, even if they were kept in the dark, emitted rays that
would fog a photographic plate. He had come upon this discovery
accidentally. Despite Becquerel's intriguing finding, the scientific
community continued to focus its attention on Roentgen's X-rays,
neglecting the much weaker Becquerel rays or uranium rays.
 HE
IGNORED URANIUM RAYS appealed to Marie Curie. Since she would
not have a long bibliography of published papers to read, she could
begin experimental work on them immediately. The director of the
Paris Municipal School of Industrial Physics and Chemistry, where
Pierre was professor of physics, permitted her to use a crowded,
damp storeroom there as a lab. HE
IGNORED URANIUM RAYS appealed to Marie Curie. Since she would
not have a long bibliography of published papers to read, she could
begin experimental work on them immediately. The director of the
Paris Municipal School of Industrial Physics and Chemistry, where
Pierre was professor of physics, permitted her to use a crowded,
damp storeroom there as a lab.
|
A
clever technique was her key to success. About 15 years earlier, Pierre
and his older brother, Jacques, had invented a new kind of electrometer,
a device for measuring extremely low electrical currents. Marie now
put the Curie electrometer to use in measuring the faint currents
that can pass through air that has been bombarded with uranium rays.
The moist air in the storeroom tended to dissipate the electric charge,
but she managed to make reproducible measurements.
“Instead of making these bodies act
upon photographic plates, I preferred to determine the intensity
of their radiation by measuring the conductivity of the air exposed
to the action of the rays.”
|
 |
|
This device for precise electrical measurement,
invented by Pierre Curie and his brother Jacques, was essential
for Marie's work. (Photo ACJC)
You can exit this site to an exhibit
on the discovery of the electron
|
|
With numerous experiments
Marie confirmed Becquerel's observations that the electrical effects of
uranium rays are constant, regardless of whether the uranium was solid or
pulverized, pure or in a compound, wet or dry, or whether exposed to light
or heat. Likewise, her study of the rays emitted by different uranium compounds
validated Becquerel's conclusion that the minerals with a higher proportion
of uranium emitted the most intense rays. She went beyond Becquerel's work,
however, in forming a crucial hypothesis: the emission of rays by uranium
compounds could be an atomic property of the element uranium--something
built into the very structure of its atoms.
 ARIE'S
SIMPLE HYPOTHESIS would prove revolutionary. It would ultimately contribute
to a fundamental shift in scientific understanding. At the time scientists
regarded the atom--a word meaning undivided or indivisible
-- as the most elementary particle. A hint that this ancient idea was
false came from the discovery of the electron by other scientists around
this same time. But nobody grasped the complex inner structure or the
immense energy stored in atoms. Marie and Pierre Curie themselves were
not convinced that radioactive energy came from within atoms--maybe, for
example, the earth was bathed in cosmic rays, whose energy certain atoms
somehow caught and radiated? Marie's real achievement was to cut through
the complicated and obscure observations with a crystal-clear analysis
of the set of conclusions that, however unexpected, were logically possible. ARIE'S
SIMPLE HYPOTHESIS would prove revolutionary. It would ultimately contribute
to a fundamental shift in scientific understanding. At the time scientists
regarded the atom--a word meaning undivided or indivisible
-- as the most elementary particle. A hint that this ancient idea was
false came from the discovery of the electron by other scientists around
this same time. But nobody grasped the complex inner structure or the
immense energy stored in atoms. Marie and Pierre Curie themselves were
not convinced that radioactive energy came from within atoms--maybe, for
example, the earth was bathed in cosmic rays, whose energy certain atoms
somehow caught and radiated? Marie's real achievement was to cut through
the complicated and obscure observations with a crystal-clear analysis
of the set of conclusions that, however unexpected, were logically possible.
Marie tested all the known
elements in order to determine if other elements or minerals would make
air conduct electricity better, or if uranium alone could do this. In
this task she was assisted by a number of chemists who donated a variety
of mineral samples, including some containing very rare elements. In April
1898 her research revealed that thorium compounds, like those of uranium,
emit Becquerel rays. Again the emission appeared to be an atomic property.
To describe the behavior of uranium and thorium she invented the word
“radioactivity” --based on the
Latin word for ray.
� 2000 -
American Institute of Physics
|

