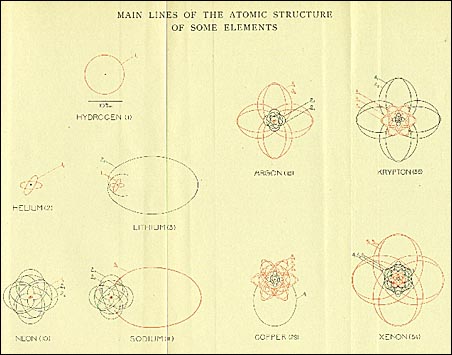
Some of the most beautifully drawn diagrams of the quantum orbits of electrons in the Bohr-Sommerfeld theory of various atoms. In the more modern view, the positions of electrons would be shown as a fuzzy cloud. From H. A. Kramers and Helge Holst, The Atom and the Bohr Theory of its Structure (New York: Alfred A. Knopf, 1926)
How are atoms structured? According to the so-called old quantum theory, first enunciated by Bohr in 1913 and elaborated by Sommerfeld three years later, atoms consist of a tiny positive nucleus surrounded by negative electrons which orbit the nucleus like planets around a sun. Nearly all the mass of the atom is concentrated in the nucleus. The number of electrons is given by the "atomic number" of the element. The electrons are held in their orbits around the nucleus by electrical attraction, similar to the gravitational attraction that holds the planets in their orbits around the sun in our solar system. But unlike our solar system, the energies of the electrons can occur only in certain fixed amounts, which correspond to certain fixed orbits. These characteristic quantities, or "quanta," of energy made this a "quantum theory" of the atom. Einstein had showed that light too could have energy only in fixed units or quanta of energy. Einstein called these "light quanta." Today they are called photons.
An electron in an atom could jump up from one fixed orbit to an orbit of higher energy, but only if it absorbed energy precisely equal to the difference of energy between the orbits. Likewise, an electron could jump down to an open lower-energy orbit by giving off energy precisely equal to the energy drop. These two events are the origins of the so-called absorption and emission spectra of the elements--their characteristic colors.
The quantum behavior of electrons in atoms contradicted not only the "classical" mechanics of Sir Isaac Newton, but also the classical electromagnetic theory, which was developed in the nineteenth century and was spectacularly successful for describing light and radio waves. Even worse, while an electron orbited in a quantum energy state, it did not radiate away its energy as the electromagnetic theory required. Instead, as Bohr postulated but could not explain, each quantum orbit could be considered a "stationary state," with energy losses or gains occurring only when the electrons jumped between the stationary states.
In 1916, Sommerfeld enhanced the Bohr theory of the atom by introducing non-circular orbits, by allowing quantized orientations of the orbits in space, and by taking into account the relativistic variation in the mass of the electron as it orbited the nucleus at high speed.
The Bohr-Sommerfeld quantum theory of the atom proved remarkably successful for the simplest case, a hydrogen atom (one electron orbiting a nucleus). Difficulties began to arise, however, for more complicated atoms in the early 1920s.

