Introduction to the History of Superconductivity
(for physics students and scientists)
by Charles Slichter
Download complete audio file (9.54MB) (Does not correspond exactly to the edited text below); or listen and read along using the embedded player below.
 |
| Charlie Slichter |
Before you hear Bob Schrieffer tell about some of the highlights of the work he did with John Bardeen and Leon Cooper - work that led to an explanation of superconductivity — I want to tell you a bit about superconductivity and why their achievement was one of the major scientific events of the 20th century.
First of all: what is superconductivity? It's an absolutely remarkable phenomenon discovered in 1911 by a student working with the famous Dutch scientist, Kamerlingh-Onnes. Kamerlingh-Onnes pioneered work at very low temperatures — temperatures just a few degrees above the absolute zero of temperature. He succeeded in reaching temperatures much colder than anyone before him, and thus opened a new frontier for science — a field of science previously unexplored, the field of low temperature physics.
He and his students set to work to study what happened to various properties of materials when they were that cold. One of his students was studying the electrical resistance of wires. He found that as he cooled mercury wire the electrical resistance of the wire took a precipitous drop when he got to about 3.6 degrees above absolute zero. The drop was enormous - the resistance became at least twenty thousand times smaller. The drop took place over a temperature interval too small for them to measure. As far as they could tell, the electrical resistance completely vanished.
 |
| H. Kamerlingh-Onnes |
To test for the complete vanishing of the electrical resistance, Kamerlingh-Onnes devised an ingenious experiment. He took a closed circle of mercury wire and caused a current to flow around the circle. With his experimental arrangement one would expect ordinarily that resistance would cause the current to die out quickly, much as friction and air resistance cause a bicycle coasting on a level road to come to a stop. He found that for a loop of mercury wire the current, once started, would persist for as long as the wire was kept cold. The persistence of the electrical current in the circuit is a kind of perpetual motion — it's a totally startling phenomenon for physicists.
Physicists understand quite well why an ordinary metal resisted the flow of electric current — why, so to speak, the electrons experienced friction in flowing through a conductor — yet something must go wrong with those ideas when the metal becomes superconducting, in order to allow the persistent seemingly-frictionless flow of current in the superconductor.
The general picture scientists had was that the resistance arises because moving electrons — which are what produce the electric current — from time to time bump into the atoms of the metal and are deflected. Thus, though they may be given an initial motion through the crystal, that motion does not persist. It's like trying to throw a baseball through a grove of trees. It bounces off the trees and comes to rest. The vanishing of electrical resistance seems analogous to requiring that the grove of trees vanish — and explaining superconductivity is like explaining why the grove appears to vanish. Remember, it's not fair for physicists to take magic as a reason! The fact is that below a certain temperature many metals enter into a new state of matter: the superconducting state. Just suppose you knew water only as a liquid - how curious you'd be when you discovered its transition into a new state of matter: ice.
You can well imagine that the explanation of this phenomenon of persistent current challenged the very best theoretical minds. Yet superconductivity remained an enigma for decades. Many of the world's greatest scientists tried to solve the mystery of the perpetual motion, but without success - at least five Nobel Prize winners. John Bardeen tried unsuccessfully shortly after finishing graduate school. [Bardeen talks about this here.] Even while working on semiconductors - and sharing in the discovery of the transistor - the challenge of superconductivity kept nagging at him in the back of his mind.
There was really no chance for any of the theorists to solve this problem at the time of discovery because before one could explain it, one had to have the quantum theory in the form that Schrödinger and Heisenberg developed, which didn't take place until the 1920's. For a long time the phenomenon of superconductivity was characterized by the statement that the electrical resistance vanished completely.
 |
| Walther Meissner |
However, in 1933 Meissner and Ochsenfeld discovered another property of superconductors, which is in fact believed by many to be an even more basic characterization. This phenomenon, which is popularly called the Meissner effect, has to do with the magnetism of a superconductor. You're no doubt familiar with the fact that iron has remarkable magnetic properties. Iron tends to draw to it the lines of magnetic force of a magnet. That's why iron is often used to make electromagnets. It helps to guide the magnetic lines of force around in space where you wish to have them. The superconductor is just the opposite. It's what is called a perfect diamagnet. A superconductor excludes the lines of magnetic force. If you bring a small bar magnet up to a superconductor, the superconductor bends the lines of force away from it and doesn't allow them to penetrate.
Around 1935 another important theoretical advance in understanding superconductivity
was made by Fritz London and his brother Heinz. In an ordinary metal we
describe the phenomenon of electrical resistance by the famous Ohm's law.
What the London brothers did was to show that there was another mathematical
relationship which should be used in place of Ohm's law to describe superconductors.
From this other relationship which they developed, they were able to explain
both the Meissner-Ochsenfeld experiment as well as the persistent current
of Kamerlingh-Onnes as two manifestations of the same thing.
I suppose in some ways the single most important experiment which directly
played a role in guiding the way to an explanation of superconductivity
was the experiment on the "isotope effect." This occurred in
1950 and, as so often happens in science, papers from two laboratories
simultaneously revealed the same results. One paper told of the work of
Reynolds, Serin, Wright and Nesbitt at Rutgers [University]. The other
was by Maxwell working at the [National] Bureau of Standards.
You know that the same chemical element may come with different nuclear
masses - so-called isotopes. What these workers did was to prepare
samples of material - in this case mercury - with their isotopic
masses varying by a few percent between different samples. They found
that the critical temperature for the superconducting transition was lower
in the sample which had the higher isotopic mass. In fact the critical
temperature was inversely proportional to the square root of the average
isotopic mass of the substance. Well, this tells you that the mass of
the nuclei was playing some role in the phenomenon of superconductivity.
Physicists are quite familiar with expressions which involve the square
root of a mass. Suppose you think of a spring with a mass attached to
it. If you give the mass a little push it will vibrate and the frequency
of that vibration goes inversely with the square root of the mass. You
may wonder what a mass and a spring has to do with superconductivity.
Well, the connection is simple. If you want to have a simple picture of
a solid you might think of it as a regular array of atoms - for example,
think of a jungle gym and think of the intersections of the points on
the jungle gym as representing the positions of the atom. In a jungle
gym the joining points of course are rigidly spaced apart by the rods
of the jungle gym, but in a solid it's probably a better approximation
to think that the atoms which are joined together are not rigidly attached,
and in fact the distance between them can be varied a little bit if you
squeeze on the solid or pull on it. It's probably a good approximation
to think of the solid as consisting of a bunch of masses - the masses
of the atom - joined together by a set of springs. If you think
of the solid in this manner then you realize that if you gave the solid
a little poke you would set all those masses jiggling and all the springs
vibrating. In any ordinary solid, this kind of jiggling phenomenon is
always present unless you are at absolute zero. It goes by the name of
the lattice vibrations.
So what the isotopic experiments we've just talked about showed was that
although the electrical conductivity was known to arise because of the
motion of the electrons, there's some role of these lattice vibrations.
They enable the electrons suddenly to move through the lattice, evidently
without hindrance, when the sample is cooled to the critical superconducting
temperature.
The next big experimental discovery was done by two groups: Goodman, who
was making thermal conductivity experiments, and Brown, Zemansky and Boorse,
who were making specific heat measurements. They discovered what is called
the energy gap. I must confess that I find explaining the energy gap the
most difficult part of the explanation of superconductivity. You have
to be patient with me while I back up a bit to get us all together in
our concepts.
You're all familiar with the way in which one builds up the periodic table by adding electrons to an atom. As one does this, one thinks of orbits of an atom which one fills with electrons. The unique chemical properties are associated with the extent to which an orbit is full or empty. Here the electrons can go only into certain orbits and only one electron can go into any given orbit. This exclusive property of electrons was first noted by Wolfgang Pauli, after whom the phenomenon is named: the Pauli Exclusion Principle. It's of great importance throughout all of physics and it plays an important role in understanding superconductors.
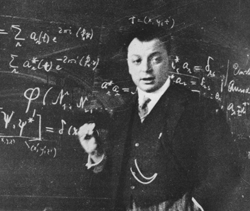 |
| Wolfgang Pauli |
Now, when we talk about a metal and we think of putting the electrons in it, we can get a pretty good picture if we think of those electrons as bouncing around inside the metal - the metal being, so to speak, like a box. We can think of the electrons very much as we think of the atoms of a gas which are bouncing around inside whatever container the gas is in. In an ordinary classical view of the world - the way people thought before the quantum theory was discovered - we would say that if we got the metal very cold, the electrons would be moving around rather slowly, and in fact they'd come to rest when one got to absolute zero. That would be a proper description of things were it not for the Pauli exclusion principal. The fact is that when electrons are in a metal, they can possess certain orbits in much the same way as electrons in an atom can possess only certain orbits. One way of thinking about these orbits is that some electrons move slowly, some move somewhat faster and some move even faster. The orbits which are possible can be specified by the speed and the direction in which the electrons are allowed to move. If we then start putting electrons into a metal to achieve the situation at absolute zero, the first electron we would put in would go into the lowest energy orbit, the next would go into a somewhat higher energy orbit, and so on until we had put in the proper number of electrons. Those last ones we put in have a good deal more energy than the first ones. The energy which they have relative to the first one is commonly called the "Fermi energy," after Enrico Fermi who first calculated its value.
Now, suppose we think about what happens in this metal if we were to
heat it a bit above the absolute zero. When you heat something, you give
a little more energy to all its parts. The electrons are no exception.
Think of one of those electrons which initially has a rather low amount
of energy. Of course, if you try to give it more energy it has a problem,
because the orbits of somewhat higher energy are already occupied by other
electrons, and the Pauli Principle does not let this electron switch over
into an orbit which is already occupied. This same argument applies to
most of the electrons. But now let's talk about those electrons which
have the Fermi energy - that is to say, they were the last ones to
get added into the energy states and the ones therefore which are moving
around most rapidly. Those electrons have nearby energy orbits which are not occupied by electrons. So if you heat the metal, they are
free to speed up a little bit and thus go into an orbit which is just
a bit above the orbit in which they used to be. There is in fact a continuous
set of energies available to those electrons at the Fermi energy, so they
can gradually add energy as the metal is warmed.
This brings us to the point of the energy gap. Suppose instead of having
the situation I've just described in which one could give those electrons
of the Fermi energy just a little bit more energy, suppose they had to
pay, so to speak, an entrance fee to gain energy. Suppose there weren't
any states which were available close by. Suppose you had to give them
a really large chunk of energy before their motion could change. Then
one has described what is called a gap in the spectrum of the possible
energy states. This is the situation which exists in superconductors.
This energy gap was discovered in the experiments of Goodman on thermal
conductivity and Brown, Zemansky and Boorse on specific heat. The experimental
evidence was clear.
It was in the early 1950's when John Bardeen decided to work again on the problem of superconductivity. By then some more clues had been found, but I won't explain them since it would take too long. But, although scientists had accumulated a number of facts about the new state of matter, no one had been able to put it all together and provide a theoretical explanation for it.
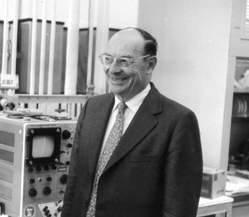 |
| John Bardeen |
Now, let's go back a bit to the discovery of the isotope effect in 1950. When John Bardeen heard about it, he was stimulated to work again on the problem of superconductivity. He had in fact worked on it at various earlier times and always kept it in the back of his mind. Meanwhile, the British physicist Fröhlich was very interested in superconductivity. He'd not known about the isotope effect, but he guessed that lattice vibrations might play a similar role. At the same time, Bardeen and Fröhlich independently put forward theories of superconductivity which later on turned out to be incorrect. However, both of them said they thought an essential portion of the problem had to do with what happened to the electrons whose energy was equal to the Fermi energy. Bardeen is such a great physicist that even when he's wrong, to some extent, he is still right for the most part. That's what we mean when we say a physicist has great physical intuition.
After these theories proved to be unsuccessful, Bardeen went back to work on further aspects of superconductivity to try to take the problem apart more thoroughly. At this point Bardeen had come to the University of Illinois from Bell Laboratories, where he, William Shockley and Walter Brattain had invented the transistor. By the way, these three men won the Nobel Prize for that invention in 1956, and in Bob Schrieffer's tape you'll notice the reference to Bardeen going off to Stockholm to receive the Prize. Bardeen was jointly a professor of physics and a professor of electrical engineering at the University of Illinois at Urbana. There, with David Pines, he studied the details of the interactions of the electrons with the lattice vibrations and with one another.
At this point Bardeen proved a tremendously important theorem. He said: suppose we consider that a superconductor is nothing but a normal metal in which we have introduced an energy gap. That is to say - instead of having some states which we know are present in a normal metal, slightly higher in energy than the energy of the electron with the Fermi energy, one would simply omit those orbits from the calculations and proceed from there. Bardeen then analyzed what happened to that metal when a magnetic field was applied to it. He succeeded in showing that he was able to derive an equation very similar to the London equation, describing the exclusion of a magnetic field by a superconductor. Bardeen made the statement at that time that if one could find the reason for the energy gap, one would very likely have the explanation of superconductivity. It's clear when one considers the papers Bardeen was writing and the thinking he was doing at that time that he was very close to the solution of the problem of superconductivity.
He had a very deep and intuitive feeling for exactly what was taking
place. He knew it involved an energy gap. He knew it involved the interactions
of the electrons with the lattice. There was one other thing which he
knew as well, and that is that superconductivity is a phase transition
of a very special kind, a type which is called a condensation in velocity
or momentum space.
So now I want to explain just what we mean by such a condensation. I can't
help but thinking, however, that you are sitting there scratching your
head and feeling, oh boy, there sure are a lot of explanations involved
in superconductivity! The fact is that this is a tough subject and you're
beginning to get a feel for why it is that people like Niels Bohr, Felix
Bloch, Richard Feynman, Werner Heisenberg, Lev Landau, the Londons, and
others who are just brilliant physicists worked on this problem all those
years and in fact didn't succeed in cracking the problem. Well, it's difficult,
and that's why we find that even an effort at an elementary description
of what happened is a pretty tough thing to listen to as well.
Phase transitions are something that we're all familiar with. I suppose
the most common ones we all know are the melting of snow or ice or the
vaporizing of water into steam. Now, usually when we think about phase
transitions, we think about condensation in real space. To illustrate
that - in the summertime we've all had the experience that if you
have a drink with ice in it, water condenses on the outside of the glass.
That's why people use coasters. What's involved is simply that the water
vapor which is always present in the air is no longer permitted to remain
there when it's in contact with something that is as cold as the surface
of the glass, and the water molecules prefer to gather together to form
the droplets of liquid on the cold surface of the glass. That's an example
of a condensation - condensation in real space - that is to
say, the molecules physically come together to form little droplets of
water. Almost all the time when physicists think about condensation, they
naturally revert to condensations in real space.
But the kind of condensation which is important for superconductivity
is the condensation in another sort of space; it is the condensation in
what one could call "velocity space." This is a slightly abstract
idea, but there are very concrete ways to illustrate it. What is meant
by a condensation in velocity space is that a whole bunch of objects assume
nearly the same velocities. Contrast that with a condensation in real
space where they assume nearly the same positions. To visualize a condensation
of velocities think for example of a playground with a whole bunch of
children playing in all different portions of it, running back and forth
and scattered around on the playground. Suppose the bell rings signifying
that recess is over. Then all the children suddenly start running towards
the doorway from all over the playground. What happens is that, although
their positions are scattered, suddenly they're all running in the same
direction. They have nearly the same velocity. Note that you can condense
their velocities without condensing their positions. Think of another
example - think of the cars driving along a superhighway, which by
and large they do at the speed limit. All up and down the superhighway
there are automobiles miles apart all going the same direction at the
same speed, and as different cars enter the superhighway from the entrance
ramps, they pick up speed until they're driving at the speed limit. And
thus we see a condensation of velocity even though the positions are widely
separated. When you think about an east-west highway you might say that
the velocity of the cars are condensed at two particular points in velocity
space, namely the speed limit going east and the speed limit going west.
That is the kind of condensation that takes place in superconductivity.
The electrons condense in velocity space. The fact that this condensation
takes place in velocity space was first recognized by Fritz London, who
pointed out that this was almost surely involved in superconductivity.
That then represented the situation in the knowledge of superconductivity
when Bardeen, Cooper and Schrieffer got together at the University of
Illinois. There were three critical elements — 1: there was a condensation
in velocity space; 2: there was an energy gap; and 3: the interactions
of the conduction. electrons with the lattice vibrations were evidently
critical in making the phenomenon occur.
I have to count it as one of the luckiest things in my life that I happened to be working as an experimenter in the field of superconductivity here at the University of Illinois back in 1955 to '57, just at the time that Bardeen, Cooper, and Schrieffer were working on the explanation of superconductivity. Bardeen's theoretical work in the early 1950's had stimulated my student Chuck Hebel and me to undertake a new kind of experiment on superconductivity. Our results were surprising, and we'd been to John to talk about them. And then, one day early in 1957, John Bardeen stopped me in the hall of the physics building. And it was clear he wanted to talk. He seemed to stand there almost for hours before he spoke. He's a quiet and a modest man. And then he said, "Well, I think we've finally figured out superconductivity." This was one of the great scientific announcements of the century. I may have been the first person apart from the three of them to know they'd actually solved it. You can imagine how excited I was!
 |
| Leon Cooper |
I'd like to tell you just a few things about this trio that cracked the problem of superconductivity. John Bardeen came to Illinois in the early 1950's from Bell Laboratories where he, Walter Brattain, and William Shockley invented the transistor. Leon Cooper had recently completed his PhD, in a totally different area of physics, and in the process he'd learned a set of mathematical techniques for what is called quantum field theory. He'd become quite expert in this and was viewed as one of the best young men in that general area. Bardeen felt it might be important to know these techniques in order to tackle the problem of superconductivity. So he invited Cooper to come to Urbana from the Institute for Advanced Study in Princeton. You could say Bardeen called in a quantum mechanic from the East.
Now, the first major breakthrough this trio made in superconductivity came from Leon Cooper. Scientists often crack a tough problem by judiciously unraveling one part of the mystery at a time. Here's where the role of judgment and physical intuition is paramount. You've got to decide which piece to tackle. Experiments on thermal conductivity and heat capacity had shown that superconductors had what is known as an energy gap. Bardeen's own work had shown that if one could understand why there was an energy gap, one would most likely be close to the heart of the explanation of superconductivity. Cooper set about trying to explain the existence of the energy gap.
 |
| J. Robert Schrieffer |
Meanwhile, Bob Schrieffer was at the University of Illinois as a graduate
student. He had completed his undergraduate studies at MIT, working in
a group of solid state physicists. When he reached graduation time, he
decided that the man he'd most like to do his graduate work with was John
Bardeen. Schrieffer began to work with Bardeen. As a warm-up, Bardeen suggested
some work on semiconductors. When it came time for a thesis, Schrieffer
chose to work on superconductivity. Bardeen suggested he familiarize himself
with the theoretical work Keith Brueckner had recently done on the nuclei
of atoms. Nuclei, like metals, consist of many particles close together
interacting strongly, so Bardeen hoped that theoretical methods helpful
to nuclear physicists might help on the problem of superconductivity.
Actually this tack did not lead Schrieffer to anything useful.
Leon Cooper was making an effort to find out why there was an energy gap.
Now, I should point out to you that if you start with a system which represents
a normal metal, and you introduce some sort of interaction which is going
to cause the transition to a superconductor, it's not easy to find a situation
in which a gap of energy occurs. So Cooper studied the general theories
of quantum mechanics to see under what circumstances gaps arose. He decided
to pick a highly simplified physical model of the system, because a real
metal has many electrons in it, and the fact there are so many particles
interacting presents overwhelming complexities. He found a very clever
way of simplifying this problem. He said: let's just consider the interactions
of two electrons. Now all the other electrons are present in the problem
and we have to take account of them, but he said the most important thing
which they did was to occupy all the low energy states - that is
to say the states which were filled up to the Fermi energy. Since those
electrons are occupying those states, those states were not available
in any way for the two electrons whose interactions he wished to study.
He then examined what happened to these two
electrons, taking into account two aspects which Pines and Bardeen had
delineated. The first was that the electrons repelled one another, because
they're particles of the same charge. The second thing was that the electrons
are moving through this lattice, which contained the positive nuclei with
their masses kept apart by their springs. As we mentioned previously,
it's not proper to think of the lattice as being totally rigid. Instead,
when you bring an electron in between two of the positive ions, these
ions are attracted to the electron and thus pull somewhat closer together
than they would be if the electrons were not there. When one electron
was there and the ions pulled together, it made that point in space somewhat
more favorable for a second electron to be there also. Since the pulling
together was due to one electron, one could say that in this way one had
an interaction of one electron with another by means of the lattice, and
that the interaction was energetically favorable - that is to say,
it was an attraction.
Cooper succeeded in solving the problem of the electrons interacting in
the two ways I've described. He found that there was a delicate balance
between repulsion of the electrons because they are of the same electrical
charge, and the attraction brought about by the lattice distortions we've
just described, and that when the lattice distortion term was somewhat
larger, the two electrons had a net attraction and an energy gap was formed.
Thus he was led to the conclusion that superconductivity arose when the
attractive interaction of one electron for the other, through the lattice,
was larger than the direct repulsion. This then became a criterion for
superconductivity. This paper was published in 1956 and is one of the
famous papers in the history of superconductivity. The interacting pairs
of electrons have since been known as "Cooper pairs" after their
discoverer.
Important as the step was which Cooper made, one must understand that
a large amount of the problem yet remained unsolved, because he had considered
the interaction of only a single pair of electrons, whereas in a real
metal there are many interacting electrons - something like 1023.
At this point Bardeen, Cooper and Schrieffer set about trying to generalize
Cooper's results to the problem of many interacting electrons. That is
- to make a many-body theory out of it. Now, the trouble was that
when they tried to put together solutions, they would find that although
they could make two electrons interact favorably with one another, quite
typically what that did was to make them interact with a third or fourth
electron unfavorably. The problem is somewhat analogous to one of those
complicated three-dimensional puzzles which one attempts to assemble.
When you try to put the puzzle together you may find you may have a couple
of pieces that fit, but then when you try to get the third piece in, it
won't fit, and the two pieces you already have interfere with adding it.
What you have to do is find how to fit those pieces together in just such
a way that they all simultaneously go together in a favorable manner.
That was exactly the problem that was posed to Bardeen, Cooper and Schrieffer.
The break came when Bob Schrieffer succeeded in guessing, in essence,
the nature of the solution at absolute zero. The form of the solution
which he found turned out to be especially simple when expressed in a
highly ingenious mathematical form. You can well imagine the feverish
activity which then followed as the three attempted to generalize the
solution to the higher temperatures, and to show that in fact they could
account for all of the facts of superconductivity. Schrieffer in his description
tells about their work carrying through to the final solution. The theory
was published in the spring of 1957. This theory accounted for essentially
all of the known experimental facts of superconductivity.
The trio then felt they were hot on the trail. But they still had lots to do. It's like assembling one of those three-dimensional puzzles. They knew how to handle any two electrons — but a metal has many more than just two. But within one year they were successful. They understood that a single Cooper pair was unstable. That is, the other electrons in the neighborhood would want to pair off, too. At the critical temperature — in their full explanation — the other electrons all do so. And that constitutes the phase transition from a normal metal into a superconductor.
How was it greeted? Experimenters greeted the theory with great acclaim, because it had such success in explaining their experimental results. The theory created a great flurry among the theorists as well. It's interesting however, and I think it illustrates the true nature of science, that many of the theorists felt a surge of disappointment that someone else solved this exciting problem. I remember, in fact, riding in an automobile from New Hampshire to Boston, returning from a conference on solid state physics. In the car with us was one of the truly great physicists of the time who'd worked on the problem, and he told of the enormous disappointment he felt when he found that someone else solved the problem. That led to a conversation in the car in which various people recollected their reactions. One member of the group told about a confession made to him by another truly great scientist, who said that when he first saw the account that Bardeen, Cooper and Schrieffer published saying they had solved the problem, he looked at it just closely enough to be able to see that it looked right, but couldn't bring himself to read the paper. He had to wait until one day when he himself had solved a particularly tough problem, and felt in a real mood of elation, to be able to bring himself to the point where he could sit down and really seriously study the Bardeen, Cooper, Schrieffer solution.
But the resistance to the theory was not solely because people were disappointed in not having solved the problem. When the theory first came out, it had some aspects which people questioned. But in a short period of time theorists were able to straighten those matters out and become satisfied that indeed the theory was correct. There's a marvelous quote which illustrates this, when David Schonberg remarked at the Cambridge [England] conference on superconductivity in 1959, "Let us see to what extent the experiments fit the theoretical facts."
One might have supposed that a theory which was as successful as this one would have closed the field and allowed physics to move on to other things. That was not the case. In fact the original work of Bardeen, Cooper and Schrieffer has been an enormous stimulus to work on superconductivity.
In 1972 John Bardeen, Leon Cooper and Bob Schrieffer got the Nobel Prize in physics for their theory of superconductivity.
 |
| Bardeen, Cooper & Schrieffer receiving honorary degrees from the University of Illinois, 1974 |
